Short chain polypeptide, application thereof as vaccine adjuvant and vaccine with short chain polypeptide serving as adjuvant
A technology of vaccine adjuvant and short peptide, which is applied in the field of short peptide, its application as a vaccine adjuvant, and the field of vaccines using the short peptide as a vaccine adjuvant, which can solve the problem of inability to form hydrogel and vaccine adjuvant to wrap antigen And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0052] Preparation of vaccine vac-1 loaded with OVA protein in short peptide hydrogel at pH 7.4 and room temperature 20°C
[0053] (1) Synthesis of D-configuration short peptide Fbp-G by FMOC-solid-phase synthesis method D f D f D Y, the structural formula is as follows:
[0054]
[0055] Specific steps are as follows:
[0056] 1) Weigh 0.5mmol 2-cl-Trt resin into a solid phase synthesizer, add 10mL of anhydrous dichloromethane (hereinafter referred to as DCM), place on a shaker and shake for 5min to fully swell the 2-Cl-Trt resin ;
[0057] 2) Remove the DCM from the solid-phase synthesizer equipped with 2-Cl-Trt resin with ear washing ball;
[0058] 3) Dissolve 0.75 mmol of Fmoc-protected amino acid in 10 mL of anhydrous DCM, add 0.75 mmol of DIEPA, then transfer to the above-mentioned solid-phase synthesizer, add 0.75 mmol of DIEPA, and react at room temperature for 1 h;
[0059] 4) Sealing: Remove the reaction solution in the solid-phase synthesizer with ear wash ...
preparation Embodiment 2
[0069] Short peptide hydrogel Car-G at pH 7.4 and room temperature 20°C D f D f D Preparation of vaccine vac-2 loaded with OVA protein
[0070] (1) Synthesis of D-configuration short peptide Car-G by FMOC-solid-phase synthesis method D f D f D Y, the structural formula is as follows:
[0071]
[0072] The specific steps are as described in Preparation Example 1, and the end-capping group is carprofen.
[0073] Its structural characterization data are as follows:
[0074] 1 H NMR (400MHz,DMSO)δ11.32(s,1H),9.19(s,1H),8.56–8.30(m,2H),8.20–7.96(m,3H),7.86(d,J=8.3Hz, 1H), 7.51–7.30(m, 3H), 7.26–7.01(m, 11H), 6.89(t, J=6.3Hz, 2H), 6.65(d, J=8.3Hz, 2H), 4.62–4.35(m ,3H),3.83–3.41(m,4H),2.98(dd,J=9.7,4.0Hz,1H),2.78–2.56(m,3H),2.33(t,J=11.5Hz,1H),1.35( d,J=5.8Hz,3H).
[0075] (2) Take 1mg Car-G D f D f D Put Y in a 1.5 ml glass bottle, add 400 microliters of PBS solution (pH=7.4), adjust the pH value to 7.4 with sodium carbonate solution, heat to boiling to completely ...
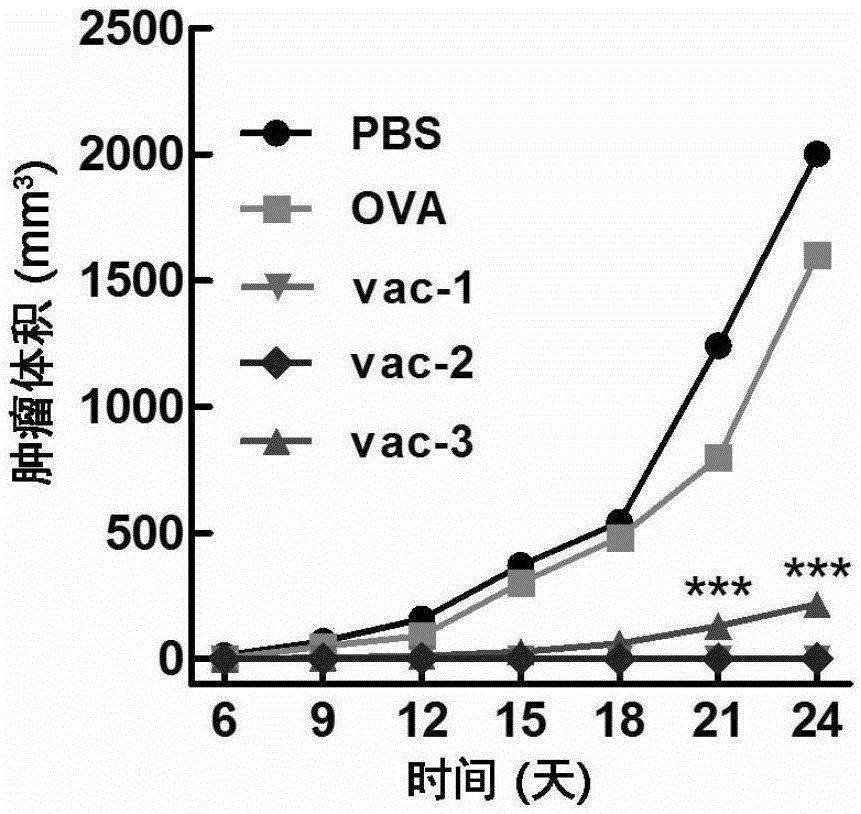
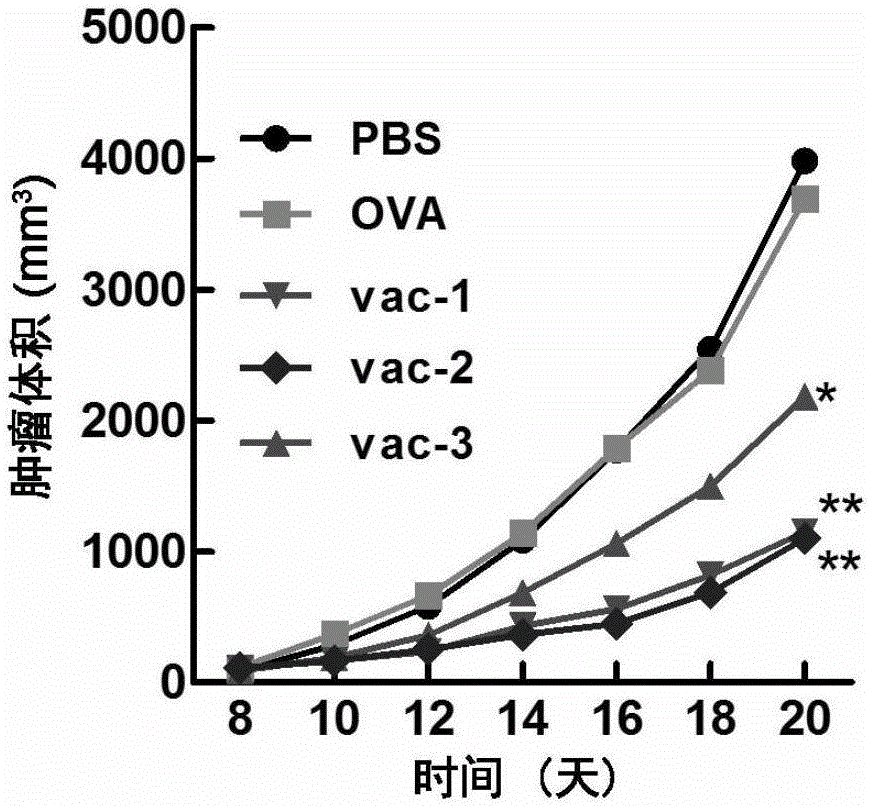
Embodiment 1
[0091](1) Mice were immunized for the first time
[0092] Take a mouse of 6-8 weeks, record the time of the first injection as 0 day, take the protein prepared in Preparation Example 1, Preparation Example 2, Comparative Preparation Example 1, Comparative Preparation Example 2 and Comparative Preparation Example 3 Vaccines vac-1, vac-2, OVA, Al-OVA, and vac-3 were dispersed into a viscous solution by vortexing the hydrogel, and then administered subcutaneously at the groin of each mouse at a dose of 100 microliters per mouse. injection.
[0093] (2) Second immunization of mice
[0094] At the time point of the 14th day, the protein vaccines vac-1, vac-2, OVA, Al -OVA and vac-3 were dispersed into a viscous solution by vortexing the hydrogel, and then subcutaneously injected into the groin of each mouse at a dose of 100 microliters per mouse.
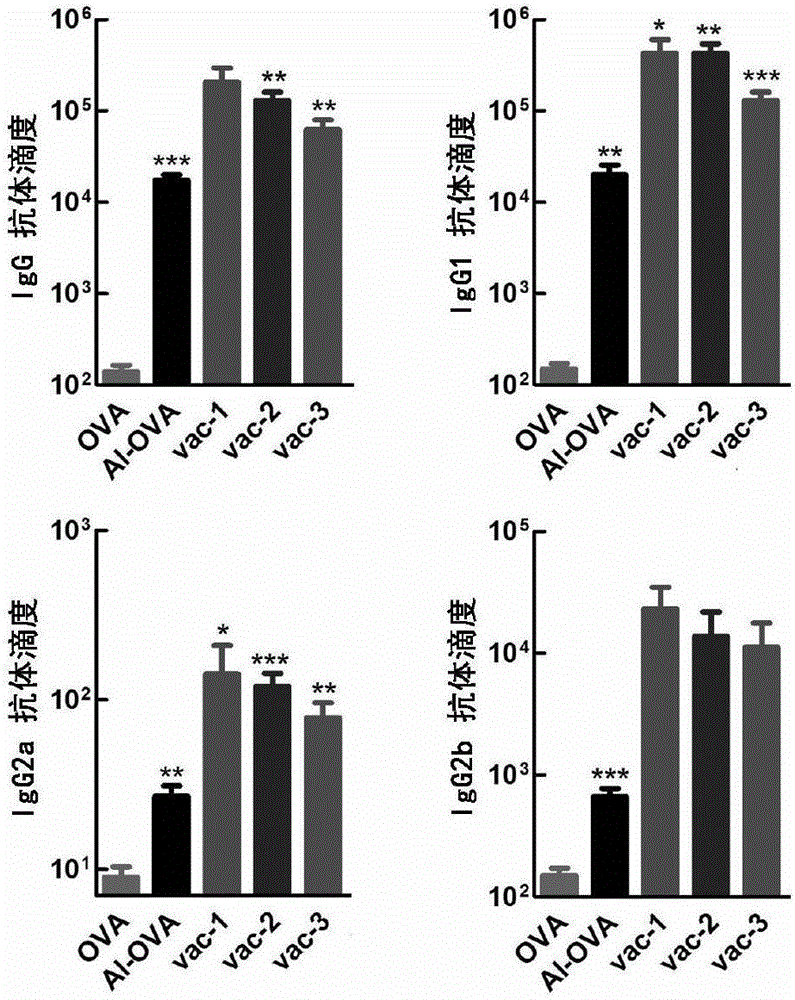
[0095] (3) Measurement of antibody titer
[0096] On the 21st day, the mouse serum was taken, and the corresponding antibody titer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com