Insulin analogues containing a glucose-regulated conformational switch
An insulin analog and insulin technology, which can be applied to insulin, peptides containing affinity tags, and medical preparations containing active ingredients, etc., and can solve the problems of decreased and increased activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
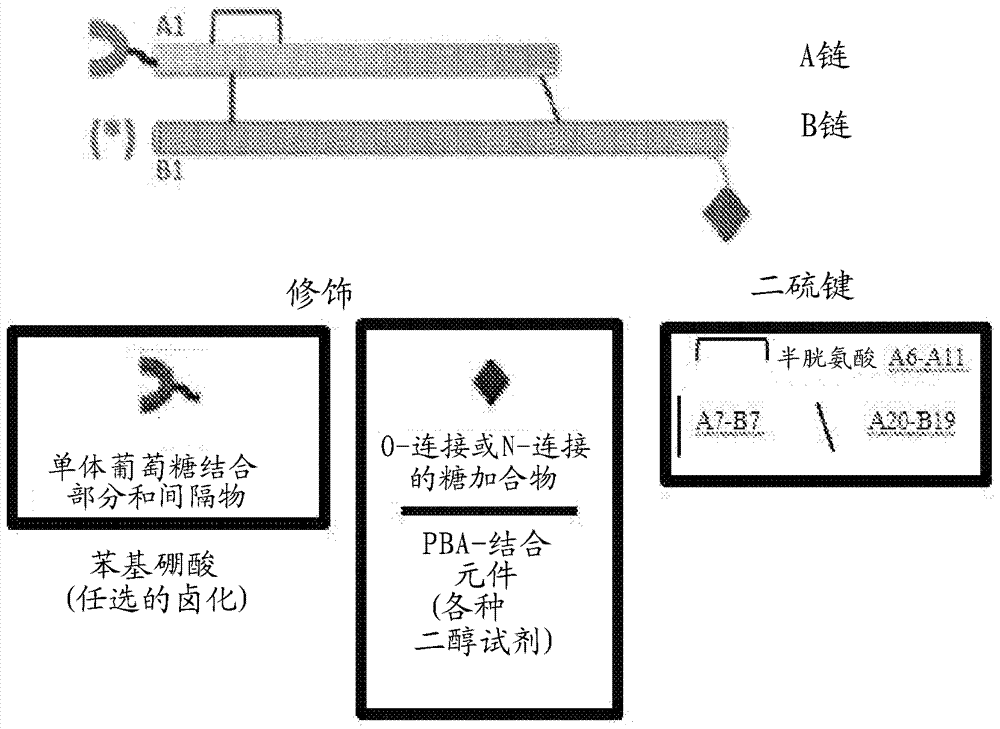
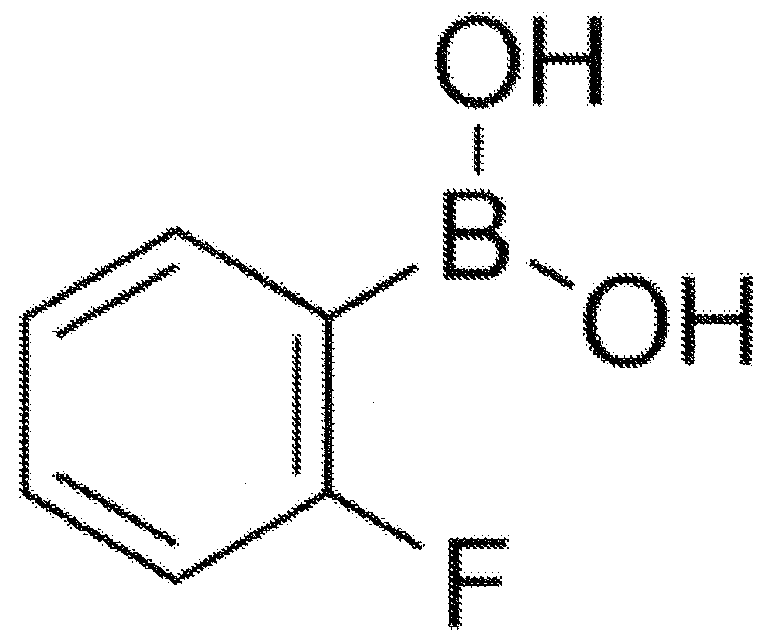
[0037] The present invention relates to an insulin analog that provides enhanced insulin resistance through a glucose-dependent rate of disassembly of insulin hexamers in subcutaneous depots and / or glucose-dependent binding to insulin receptors in the bloodstream and at target tissues. Blood sugar control in the body. Six novel insulin analogs, listed in Table 2, were prepared. Each employs fluoro-phenylboronic acid as the glucose sensing element. Fluoro-PBA moieties and Gly A1 The chemical bonds between the α-amino groups such as Figure 7 shown in; in Phe B1 The corresponding bond (not shown) is employed in the analogue with the fluoro-PBA adduct at the α-amino group of .
[0038]Two analogs contained one such adduct (α-amino group at the A chain; Table 2A), and three analogs contained two such adducts (α-amino group at both the A and B chains). amino group; Table 2B). A protocol for the derivatization of insulin fragments by the active agent FPA reagent is provided be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com