A kind of preparation method of active site materials such as p25 loaded molecular state cobalt/nickel
An active site, molecular state technology, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve problems such as poor dispersion, low performance of transition metal cocatalysts, and high price of precious metal cocatalysts. , to achieve the effect of low equipment requirements, low price and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
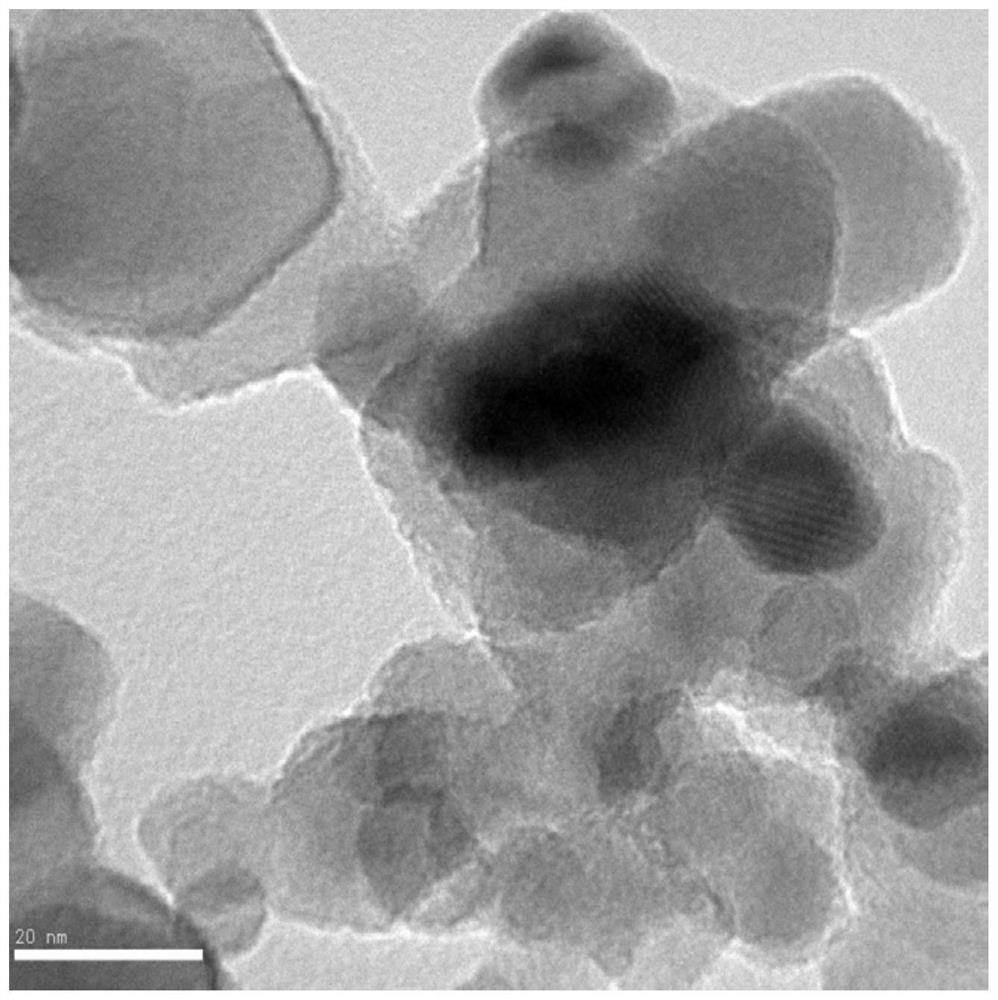
[0019] figure 1 It is the HRTEM picture of P25-Co-350 in Example 1 of the present invention, with P25 as the carrier, and ethylenediamine-N, N, N', N'-cobalt (II) tetraacetate disodium salt tetrahydrate as the precursor , to obtain P25-loaded molecular cobalt active sites.
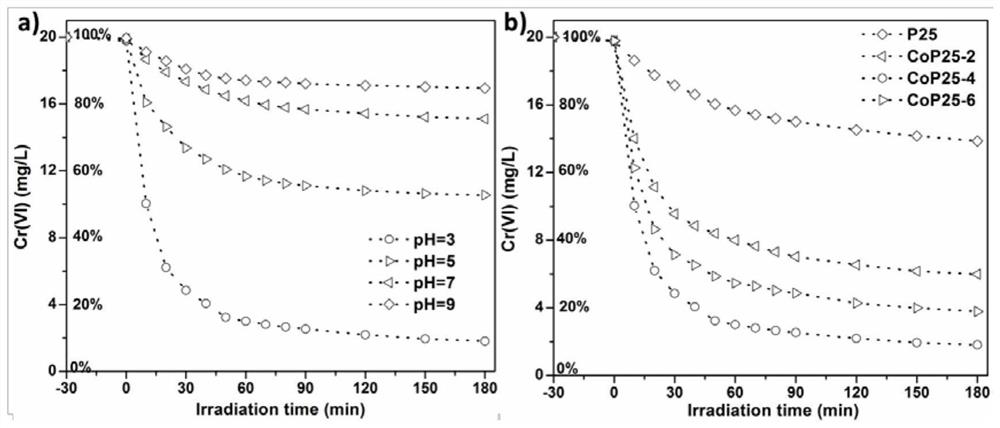
[0020] Take 0.35g of P25, dissolve it in 35mL of methanol, stir for 1 hour, then add 35mg of ethylenediamine-N,N,N',N'-tetraacetate cobalt(II) disodium salt tetrahydrate, maintain magnetic stirring at 40 degrees Celsius until The solvent was completely evaporated, and the solid was collected and roasted at 350°C for 1 hour (heating rate 1K / min), then cooled to normal temperature at 2K / min. The resulting cobalt molecular state modified HRTEM of P25 is as follows figure 1 As shown, the surface is uniform without nanoparticle formation. Its catalytic activity is figure 2 .
Embodiment 2
[0022] Using P25 as the carrier and ethylenediamine-N,N,N',N'-tetraacetate nickel(II) disodium salt tetrahydrate as the precursor, the P25-loaded molecular nickel active sites were obtained.
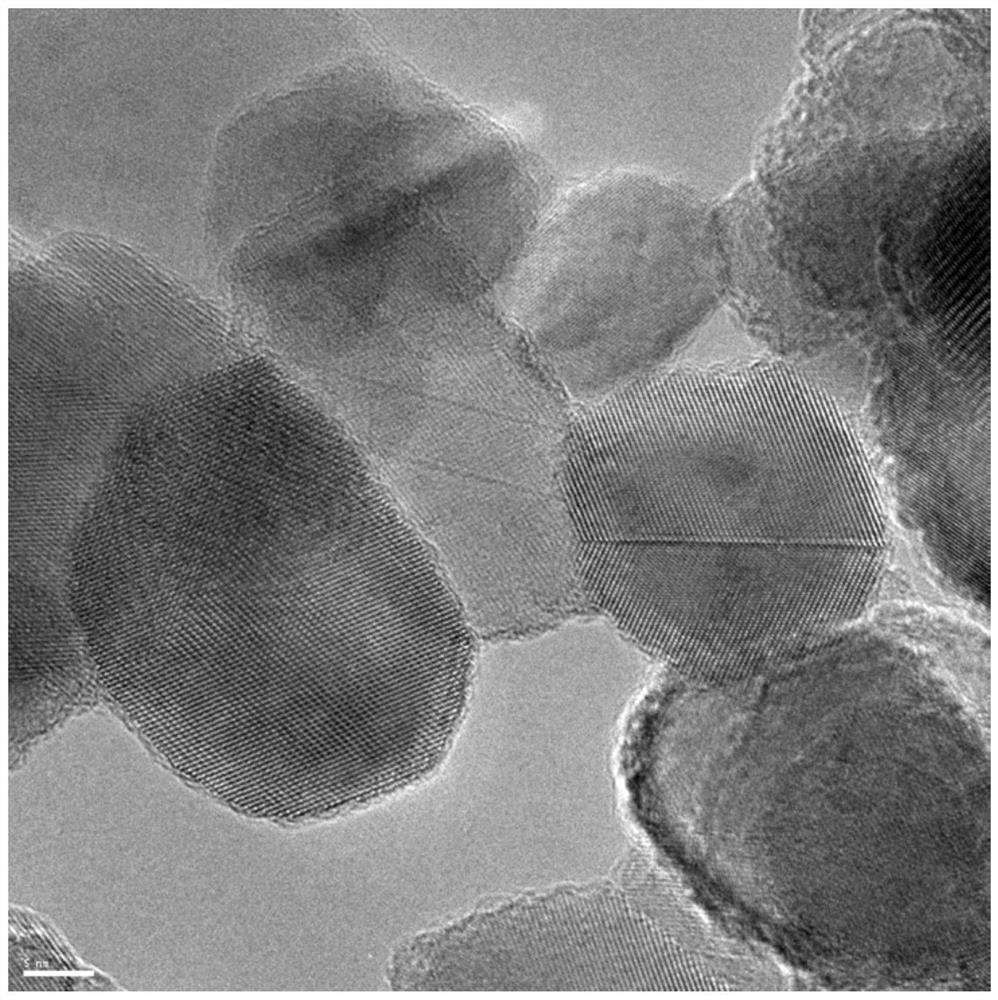
[0023] Take 0.35g of P25, dissolve it in 35mL of ethanol, stir for 1 hour, then add 35mg of ethylenediamine-N,N,N',N'-tetraacetate nickel (II) disodium salt tetrahydrate, maintain magnetic stirring at 40 degrees Celsius until The solvent was completely evaporated, and the solid was collected and roasted at 350°C for 1 hour (heating rate 1K / min), then cooled to normal temperature at 2K / min. The obtained nickel molecular state modified HRTEM of P25 is as follows image 3 As shown, the surface is uniform without nanoparticle formation.
Embodiment 3
[0025] Take 0.35g of P25, dissolve it in 35mL of methanol, stir for 1 hour, then add 35mg of ethylenediamine-N,N,N',N'-tetraacetate cobalt(II) disodium salt tetrahydrate, maintain magnetic stirring at 40 degrees Celsius until The solvent was completely evaporated, and the solid was collected, and after roasting at 400°C for 1 hour (heating rate 1K / min), it was cooled to normal temperature at 2K / min. The surface of the obtained cobalt molecularly modified P25 is uniform without the formation of nanoparticles. Its HRTEM as Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com