Method and device for preparing caprolactam through Beckmann rearrangement of cyclohexanone oxime under catalysis of TFA (trifluoroacetic acid)
A technology for the rearrangement of cyclohexanone oxime and Beckmann catalyzed by trifluoroacetic acid, which is applied in the direction of organic chemistry, can solve the problem of by-product large ammonium sulfate, and achieve the effect of high concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
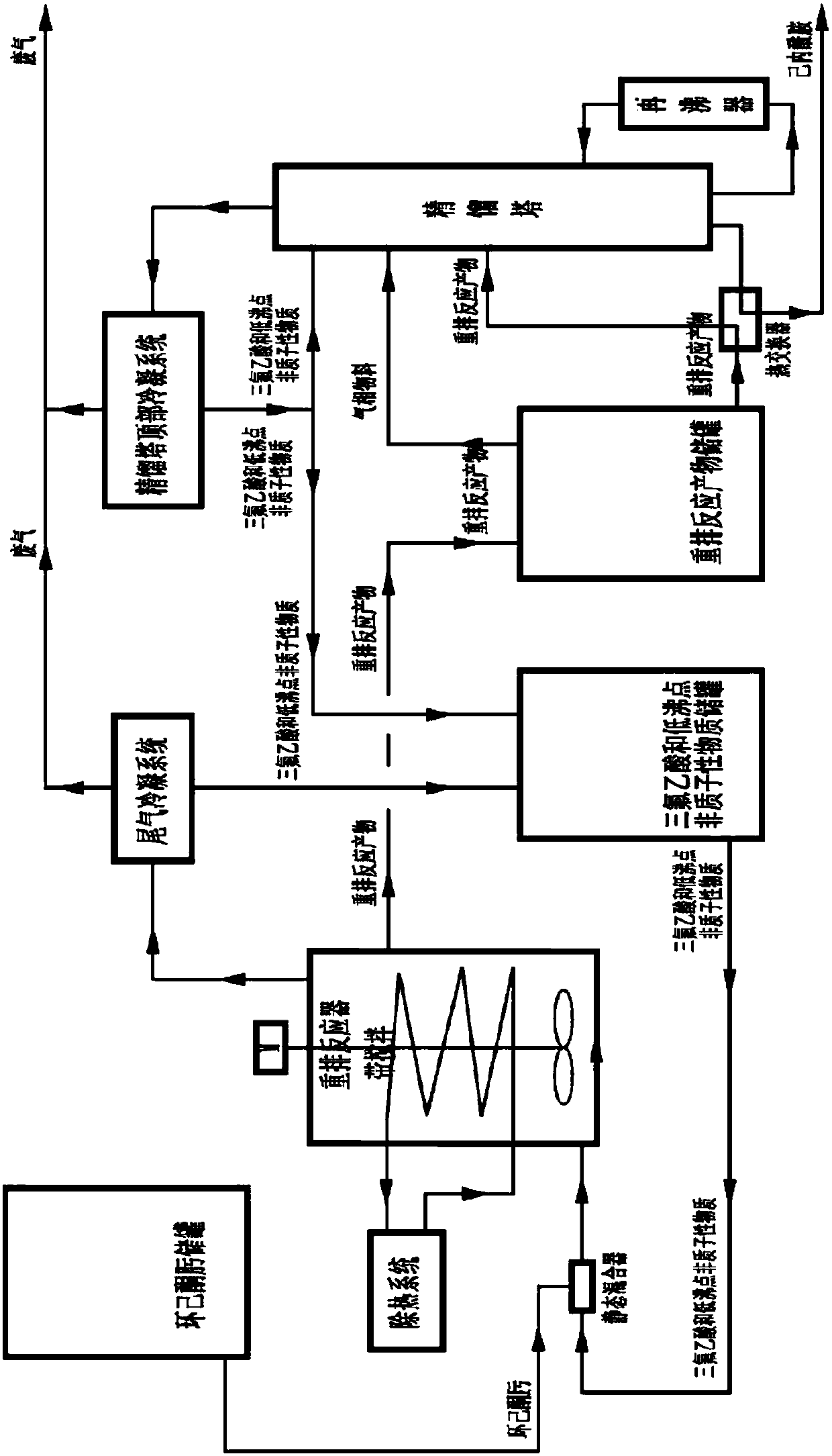
[0054] use as figure 1 The process shown in the experiment was carried out.
[0055] Acetonitrile and trifluoroacetic acid from trifluoroacetic acid and low-boiling aprotic substance storage tank were pressurized by a feed pump (not shown) at acetonitrile concentration of 0.3 wt%, and cyclohexanone oxime from cyclohexanone oxime storage tank was pressurized. Ketoxime (concentration after mixing with acetonitrile and trifluoroacetic acid is 1.9mol / L) is fully mixed in the static mixer, enters the ring distribution pipe at the bottom of the rearrangement reactor, is further fully mixed by the agitator, and Under the action of acid catalysis, the Beckmann rearrangement reaction is carried out; since the rearrangement reaction is an exothermic reaction, a cooling coil is installed inside the reactor, and cooling water is supplied to the inner coil through the cooling desalted water pump of the heat removal system, and the amount of cooling water is controlled The reaction tempera...
Embodiment 2
[0059] use as figure 1 The process shown in the experiment was carried out.
[0060] Acetonitrile and trifluoroacetic acid from trifluoroacetic acid and low-boiling aprotic substances storage tank were pressurized by a feed pump (not shown) at acetonitrile concentration of 5 wt%, and cyclohexanone from cyclohexanone oxime storage tank Oxime (concentration after mixing with acetonitrile and trifluoroacetic acid is 1.9mol / L) is fully mixed in the static mixer, and then enters the annular distribution pipe at the bottom of the rearrangement reactor, and is further fully mixed by the agitator, and in the acid Under the action of catalysis, the Beckmann rearrangement reaction is carried out; since the rearrangement reaction is an exothermic reaction, a cooling coil is installed inside the reactor, and cooling water is supplied to the inner coil through the cooling desalted water pump of the heat removal system, and the reaction is controlled by the amount of cooling water The temp...
Embodiment 3
[0064] use as figure 1 The process shown in the experiment was carried out.
[0065] Acetonitrile and trifluoroacetic acid from trifluoroacetic acid and low-boiling aprotic substances storage tank were pressurized by a feed pump (not shown) at acetonitrile concentration of 0.1 wt%, and cyclohexanone oxime from cyclohexanone oxime storage tank was pressurized. Ketoxime (concentration after mixing with trifluoroacetic acid and acetonitrile is 3mol / L) is fully mixed in the static mixer, enters the annular distribution pipe at the bottom of the rearrangement reactor, and is further fully mixed by the agitator, and in the acid Under the action of catalysis, the Beckmann rearrangement reaction is carried out; since the rearrangement reaction is an exothermic reaction, a cooling coil is installed inside the reactor, and cooling water is supplied to the inner coil through the cooling desalted water pump of the heat removal system, and the reaction is controlled by the amount of coolin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com