A tacrolimus separation and purification method
A tacrolimus, separation and purification technology, applied in the field of biopharmaceuticals, can solve the problems of drug efficacy, side effects of patients, unsatisfactory tacrolimus purity, etc., to achieve the effect of improving drug efficacy and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for separating and purifying tacrolimus shown in this embodiment comprises the following steps:
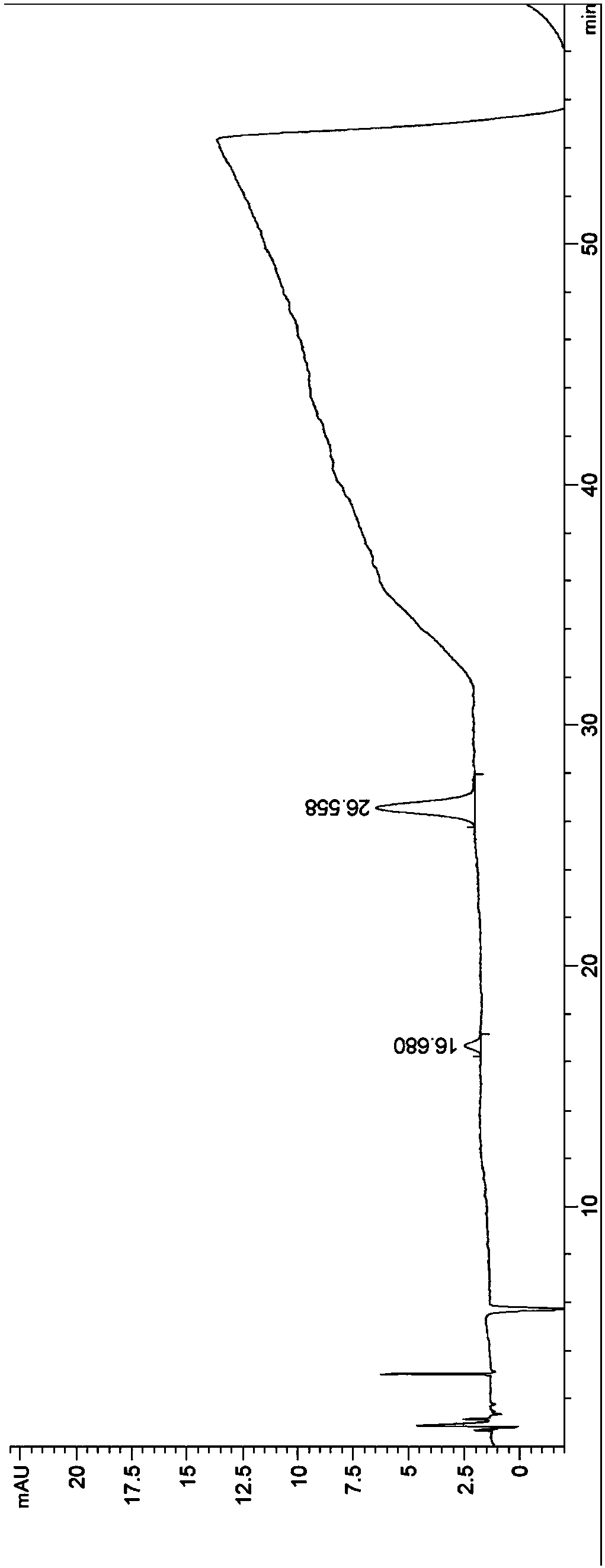
[0028] Step (1): Prepare 250 g of tacrolimus fermentation extract with low chromatographic purity, filter it with a 0.45-micron microporous organic filter, and set aside. Among them, the chromatographic purity of the tacrolimus fermentation extract is about 60%.
[0029] Step (2): In the first set of medium-pressure chromatography equipment, chromatography silica gel is used as filler, and ethanol is used for cleaning and balancing. The particle size of the chromatographic silica gel in the step (2) is 300 mesh, and the pressure condition is 60 psi. In this example, macromolecular impurities such as polysaccharides and proteins in the low-chromatographic purity tacrolimus fermentation extract were effectively removed by the first set of medium-pressure chromatography equipment, so as to facilitate further purification operations in the later stage.
[0030] Step ...
Embodiment 2
[0038] A method for separating and purifying tacrolimus shown in this embodiment comprises the following steps:
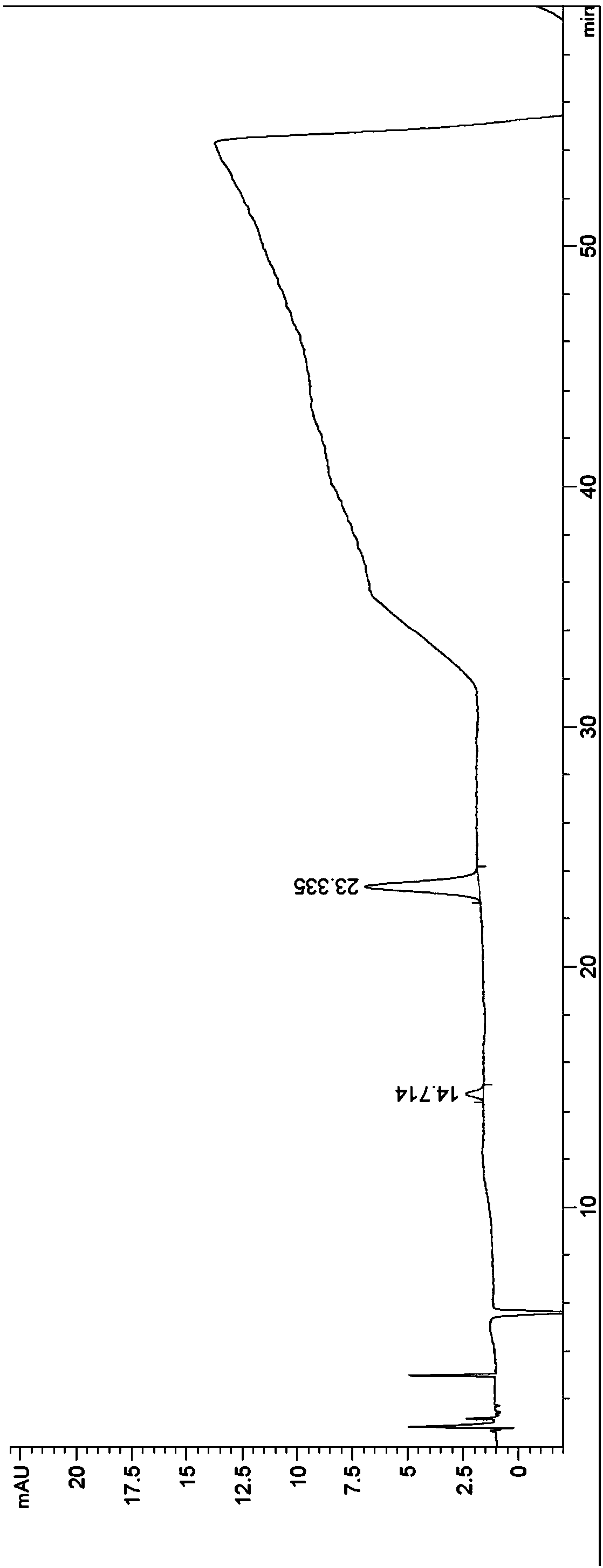
[0039] Step (1): Prepare a tacrolimus fermentation extract with low chromatographic purity, filter it with a 0.45-micron microporous organic filter membrane, and set aside. Among them, the chromatographic purity of the tacrolimus fermentation extract is about 60%.
[0040] Step (2): In the first set of medium-pressure chromatography equipment, chromatography silica gel is used as filler, and acetone is used for cleaning and balancing. The particle size of the chromatographic silica gel in step (2) is 400 mesh, and the pressure condition is 90psi.
[0041] Step (3): The low chromatographic purity tacrolimus fermentation extract is chromatographed in the first set of medium-pressure chromatography equipment, and an organic solvent (such as: ethanol) is used for elution, and the eluate is collected, and the eluate is Rotary evaporation, dissolving with organic solve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com