Pharmaceutical compound preparation for treating hyperparathyroidism
A technology of compound preparations and parathyroid glands, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., can solve the problems of patients' depression and serious complications, and achieve fewer sequelae, convenient administration, and safe use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
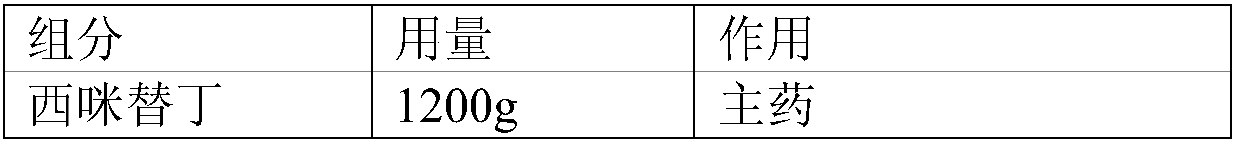
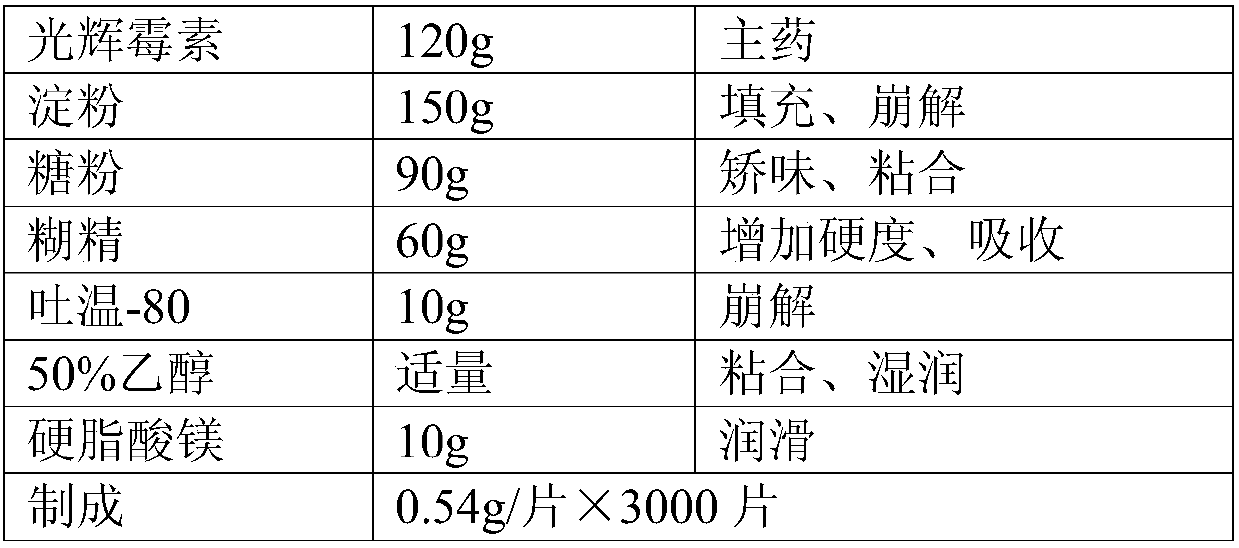
[0017] The compound oral preparation of this embodiment is prepared by a method known in the pharmaceutical industry, and the specific dosage of each component is shown in Table 1:
[0018] Table 1 The specific dosage of each component
[0019]
[0020]
[0021] The treatment effect of using two oral medicines of cimetidine and shimmermycin mixed at the same time shows that the effect of using cimetidine and shimmermycin is better than that of a single medicine, thus providing a reasonable combination for the development of two kinds of medicines. The compound preparation used has laid the foundation.
Embodiment 2
[0023] Validation of therapeutic effect in rat model:
[0024] Objective: To observe the effect of compound preparations of different ratios of cimetidine combined with shimmermycin on DOCA (deoxycorticosterone acetate) rats with hyperparathyroidism, so as to explore the therapeutic effects of different doses in the compound. effect.
[0025] Methods: 1200 SD rats, ♂, body weight 180-190g. After resecting the right kidney under aseptic conditions, DOCA 5mg / cattle was administered twice a week, sc, and fed with 1% sodium chloride solution; they were randomly divided into 12 groups, 8 in each group, and the specific grouping and treatment were shown in the table two.
[0026] Table 2 Animal grouping and drug formulation, administration method
[0027] model group
normal saline
Cimetidine group
Cimetidine 400 mg
Mitsumycin high group
Mithromycin 40 mg
compound group
Cimetidine 20 mg, Mithromycin 2 mg
Compound two group
C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com