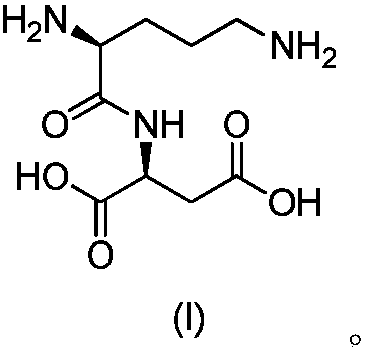
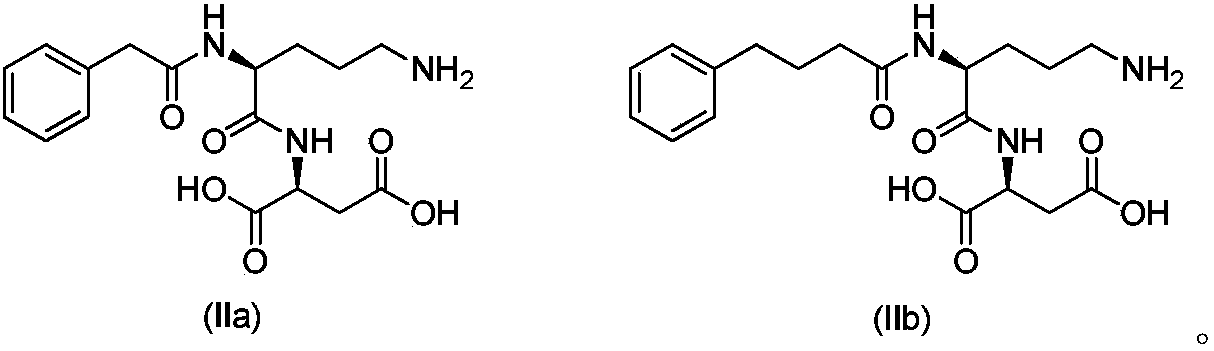
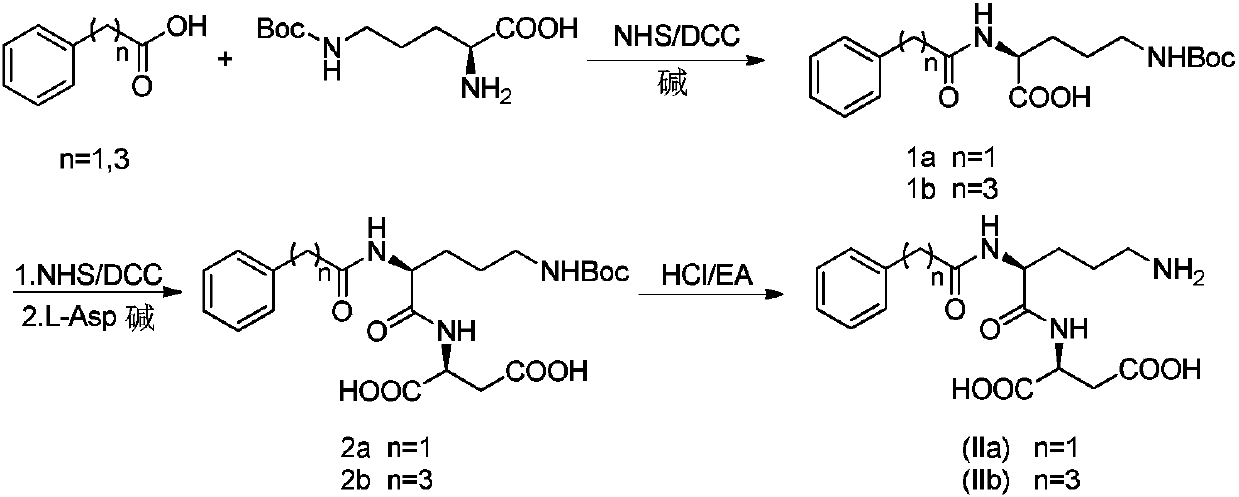
Acylated derivative for ornithine and aspartate dipeptide compound and application thereof
A peptide compound, aspartic acid technology, applied in the field of medicine, can solve the problems of undisclosed use, undisclosed ornithine and aspartic acid dipeptide compound acylated derivatives and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of intermediate 1a
[0031] Under ice-cooling, 10.892g (0.08mol) of phenylacetic acid and 9.66g (0.084mol, 1.05eq) of NHS were dissolved in 100ml of tetrahydrofuran. Another 20 g (0.097 mol, 1.2 eq) of DCC was weighed and dissolved in 100 ml of tetrahydrofuran, and added dropwise to the reaction flask. Stir the reaction for 1 hour. After the reaction is complete as monitored by TLC, add 10ml of distilled water to the reaction solution and stir for 20 minutes to quench excess DCC; filter with suction to remove insoluble matter, and the filtrate is set aside.
[0032] Weigh N-δ-Boc-L-ornithine 19.2g (0.083mol, 1.03eq) and sodium bicarbonate 13.44g (0.160mol, 2.0eq), disperse in 100ml distilled water, then add 50ml THF wherein; The filtrate obtained in the previous step was added dropwise to the reaction solution, and stirred at room temperature for 4 hours; after the completion of the reaction monitored by TLC, the pH was adjusted to pH 7 with 2N HCl ...
Embodiment 2
[0033] Example 2 Preparation of intermediate 1b
[0034] Weigh 12.697g (0.077mol) of phenylbutyric acid and 8.87g (0.077mol, 1.0eq) of NHS, dissolve it in 100ml of tetrahydrofuran, and stir in an ice bath. Another 18g (0.087mol, 1.1eq) of DCC was weighed and dissolved in 100ml of tetrahydrofuran, and added dropwise to the reaction flask. Stir the reaction for 1 hour. After the reaction is complete as monitored by TLC, add 10ml of distilled water to the reaction solution and stir for 20 minutes to quench excess DCC; filter with suction to remove insoluble matter, and the filtrate is set aside.
[0035] Weigh 19.2g (0.083mol, 1.07eq) of N-δ-Boc-L-ornithine and 13.44g (0.160mol, 2.1eq) of sodium bicarbonate, and disperse them in 100ml of distilled water, then add 50ml of THF; The filtrate obtained in the previous step was added dropwise to the reaction solution, and stirred at room temperature for 4 hours; after the completion of the reaction monitored by TLC, the pH was adjuste...
Embodiment 3
[0036] Example 3 Preparation of intermediate 2a
[0037] Weigh 7.36g (0.021mol) of intermediate 1a and 2.53g (0.022mol, 1.05eq) of NHS and dissolve in 100ml tetrahydrofuran, and stir under ice bath. Another 4.97g (0.024mol, 1.15eq) of DCC was weighed and dissolved in 100ml of tetrahydrofuran, and added dropwise to the reaction flask. Stir the reaction for 1 hour. When there is unreacted intermediate 1a in the TLC monitoring system, add an appropriate amount of DCC. When the monitoring reaction is complete, add 10ml of distilled water to the reaction solution and stir for 20 minutes to quench the excess DCC; Insoluble matter, the filtrate was set aside.
[0038] Weigh 2.942g (0.022mol, 1.05eq) of L-Asp and 7.06g (0.084mol, 4.0eq) of sodium bicarbonate and dissolve them in 150ml of distilled water. Bubbles are generated during dissolution; Stir overnight; after the reaction is monitored by TLC, adjust the pH to pH7 with 2N HCl solution, remove THF by rotary evaporation at 45°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com