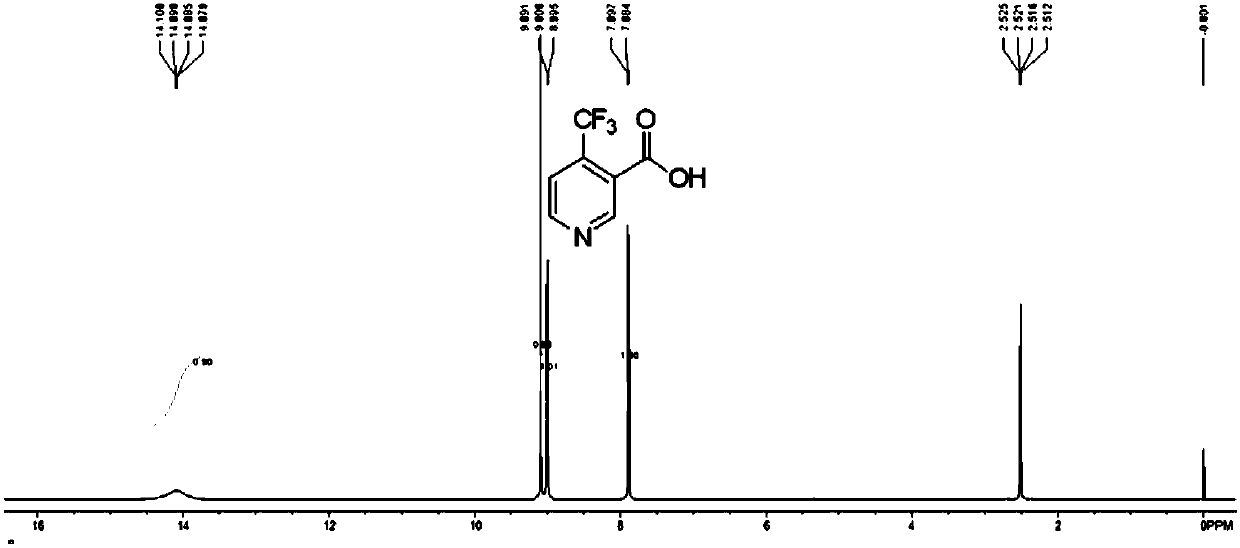
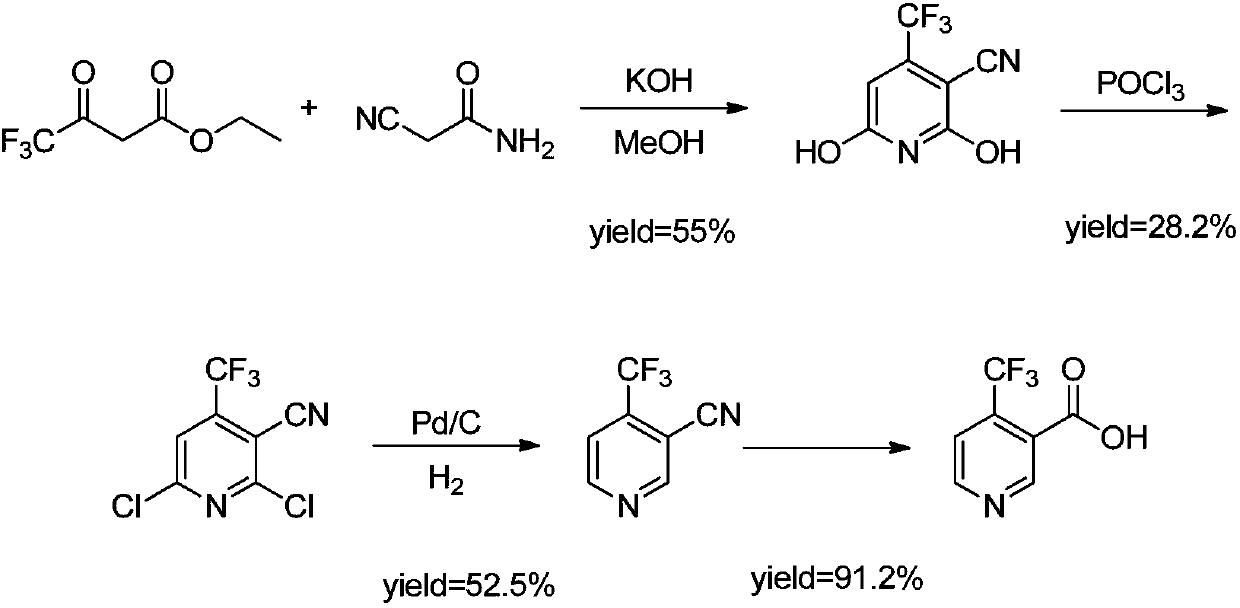
Synthesis process of 4-trifluoromethyl nicotinic acid
A technology of trifluoromethyl nicotinic acid and synthesis process, applied in the direction of organic chemistry and the like, can solve the problems of high production cost and high raw material cost, and achieve the effects of low equipment requirements, simple operation and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 12.9g (323.5mmol) of NaH (323.5mmol) and 150g of DMF into the reaction flask under the protection of nitrogen at -15~-10°C, and slowly add 4-amino-1,1,1-trifluoro-3-buten-2-one dropwise under stirring 50g (359.5mmol) solution, the system turned from colorless to light yellow and finally to red suspension, 41.75g (359.5mmol) of methyl 3-methoxyacrylate was added dropwise and reacted at this temperature for 3 hours.
[0029] Add concentrated hydrochloric acid dropwise to the reaction flask at -10°C to adjust the pH to 8-9, the system turns from dark red suspension to orange yellow, add 1.5L of water and at the same time dilute hydrochloric acid to adjust the pH to 1-2, a large amount of white flocculent solids precipitate out, stir for 0.5 hours and filter to obtain White product 81.5g (yield 88.3%).
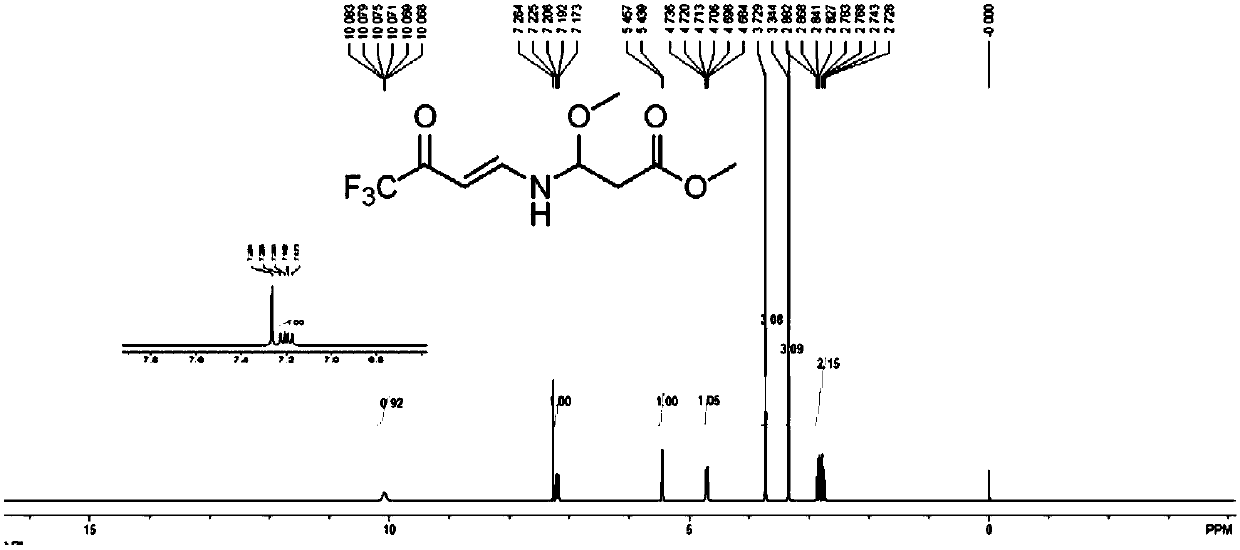
[0030] Carry out nuclear magnetic resonance analysis to the product obtained in the present embodiment, see figure 1 The spectrum shown proves that the obtained prod...
Embodiment 2
[0032] Add 7.76g (194.1mmol) of NaH (194.1mmol) and 90g of DMF into the reaction bottle under the protection of nitrogen at -15~-10°C, slowly add 4-amino-1,1,1-trifluoro-3-buten-2-one dropwise under stirring 30g (215.7mmol) and 25g (215.7mmol) of methyl 3-methoxyacrylate were mixed solution, reacted at this temperature for 3h. The system turned from colorless to light yellow and finally to a red suspension, and reacted at this temperature for 3 hours.
[0033] Add concentrated hydrochloric acid dropwise to the reaction bottle at -10°C to adjust the pH to 8-9, the system turns from dark red suspension to orange yellow, add 1L of water and at the same time dilute hydrochloric acid to adjust the pH to 1-2, a large amount of light yellow flocculent solid precipitates, stir for 0.5 hours and filter to obtain 45.2 g of light yellow product (yield 82.1%).
[0034] NMR analysis of the product obtained in this example proved that the product obtained was N-1-methoxy-2-methoxycarbonyle...
Embodiment 3
[0036] Under nitrogen protection, add 5.18g (129.4mmol) of NaH (129.4mmol) and 60g of DMF into the reaction flask at -15~-10°C and add 16.7g (143.8mmol) of 3-methoxymethyl acrylate dropwise under stirring, then slowly add 4-amino -1,1,1-Trifluoro-3-buten-2-one 20g (143.8mmol) solution, reacted at this temperature for 3 hours. The system turned from colorless to light yellow and finally to a red suspension, and reacted at this temperature for 3 hours.
[0037] Add concentrated hydrochloric acid dropwise to the reaction bottle at -10°C to adjust the pH to 8-9, the system turns from dark red suspension to orange yellow, add 0.5L of water and at the same time dilute hydrochloric acid to adjust the pH to 1-2, a large amount of light yellow flocculent solid precipitates, stir for 0.5 hours After filtration, 28.7 g (yield: 78.2%) of a pale yellow product was obtained.
[0038] NMR analysis of the product obtained in this example proved that the product obtained was N-1-methoxy-2-met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com