Method for determining methyl p-toluenesulfonate in medicine
A technology of methyl toluenesulfonate and medicine, which is applied in the field of methyl p-toluenesulfonate content, can solve problems such as difficult to achieve accurate measurement, thermal instability, decomposition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
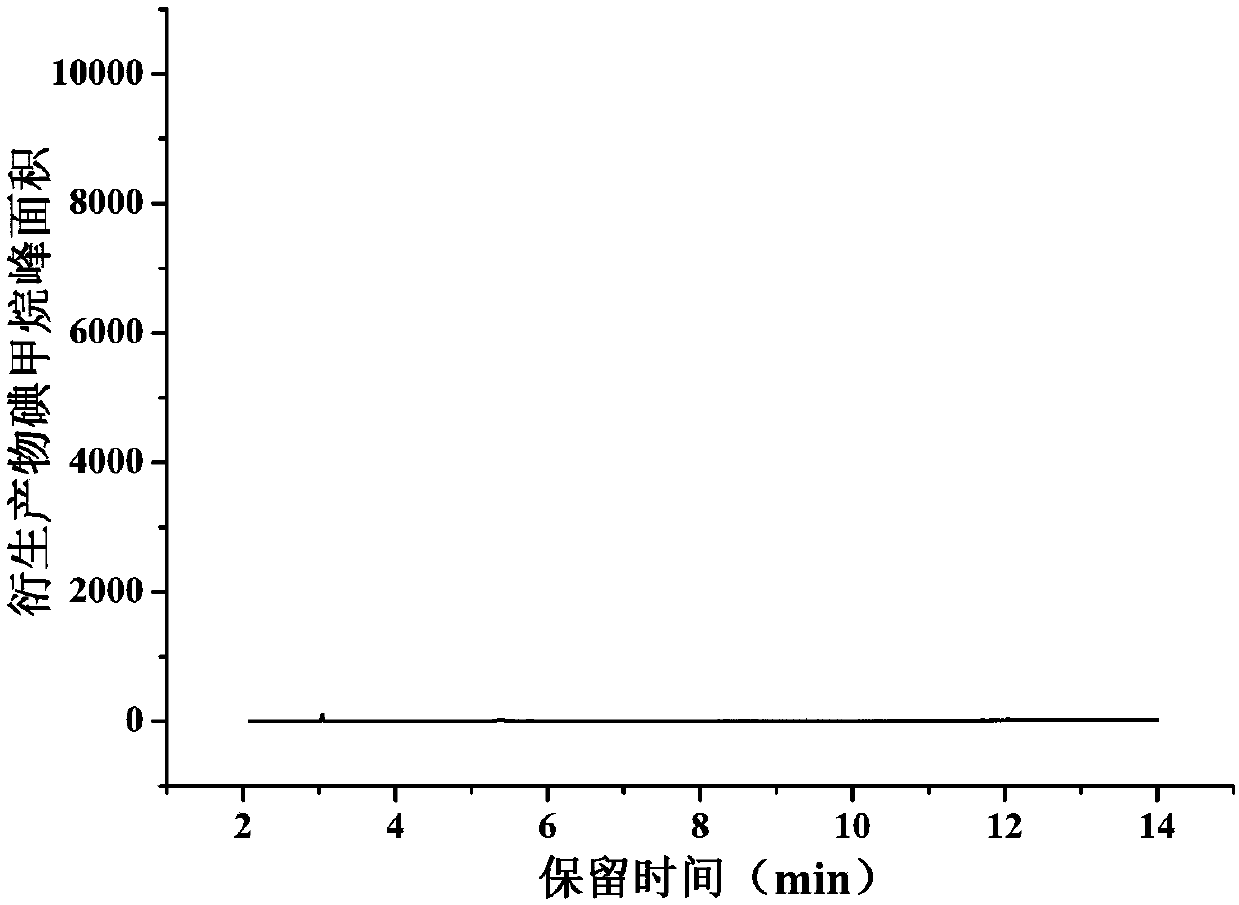
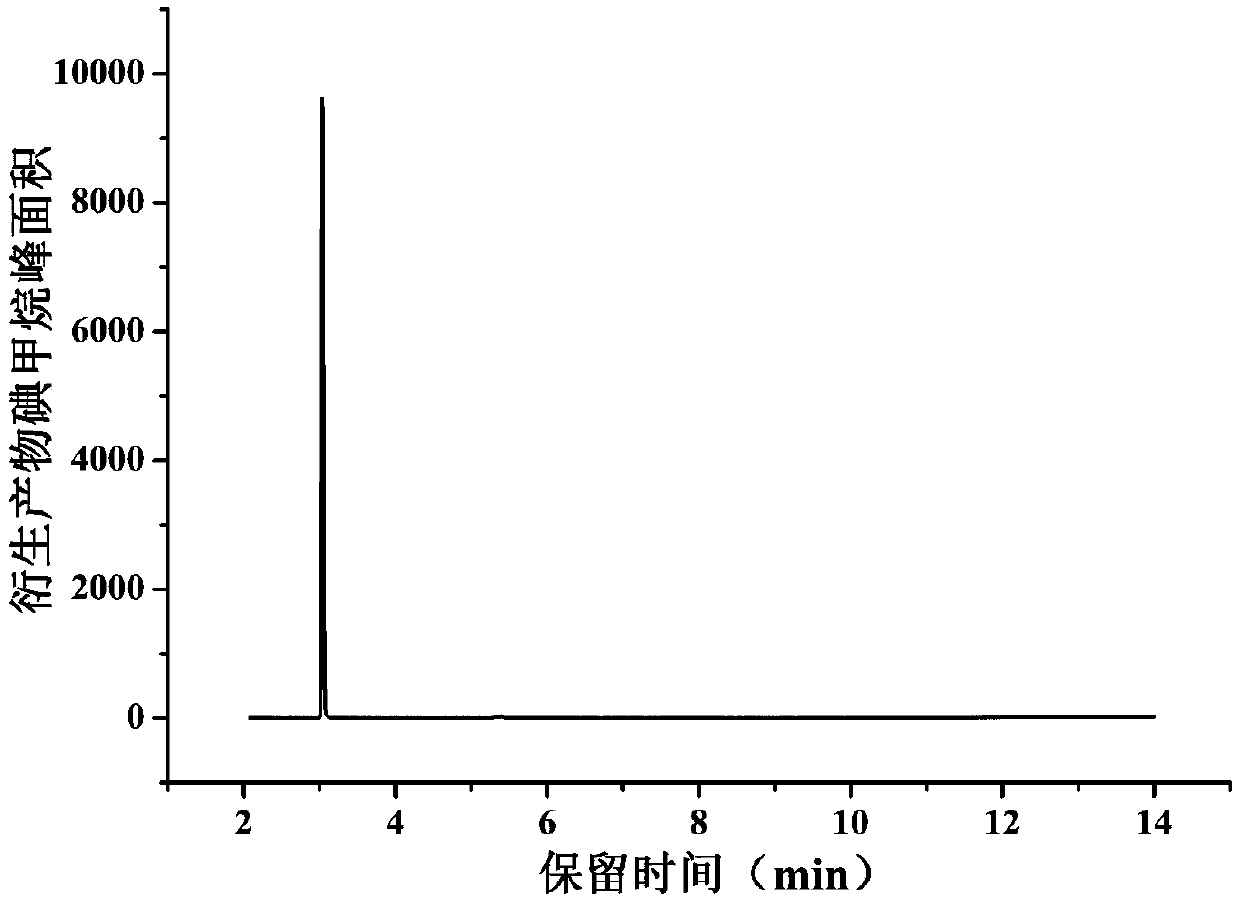
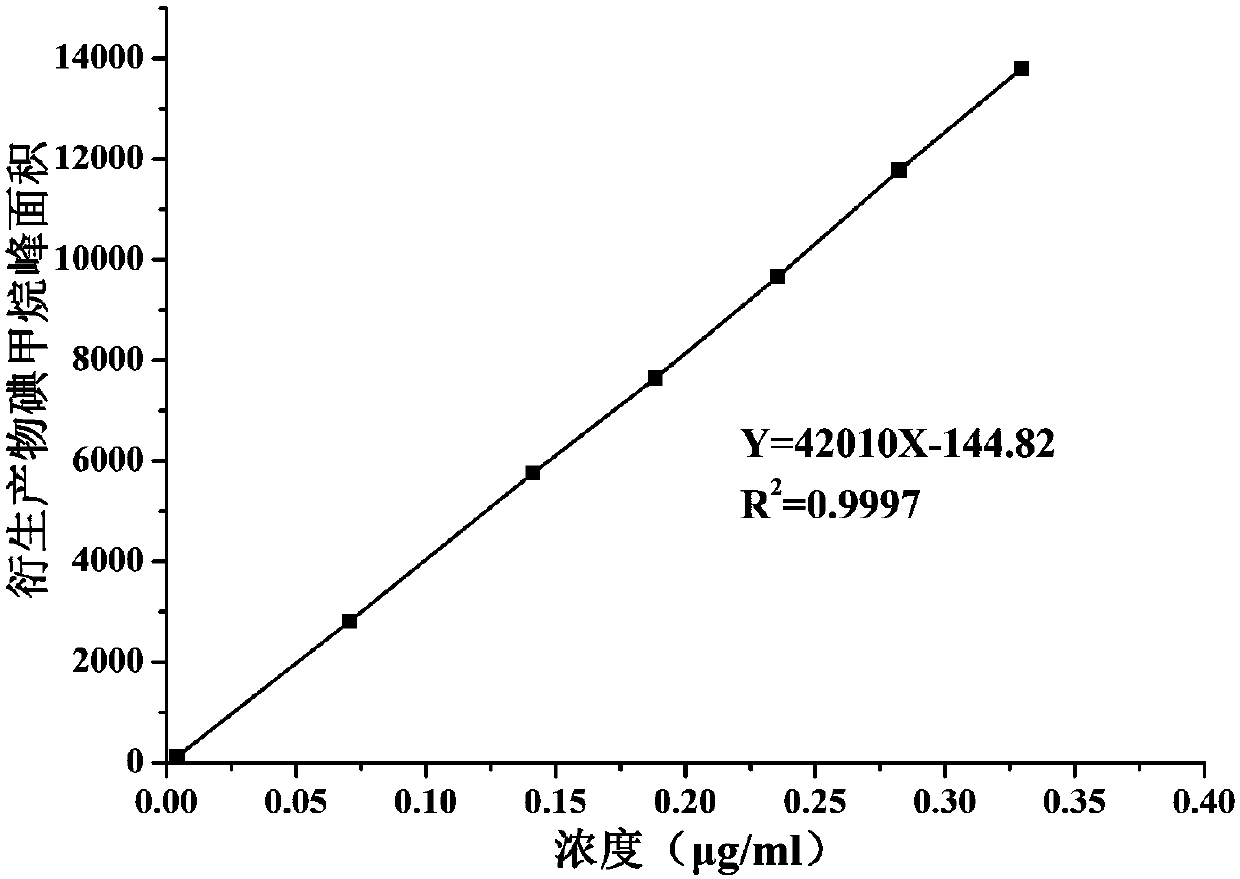
[0031] Determination of methyl p-toluenesulfonate content in artemisinin analogues
[0032] 1. Chromatographic conditions
[0033] Instruments: Agilent 7890B Gas Chromatograph - 5977A Mass Spectrometer - 7697A Headspace Sampler
[0034] Chromatographic column: DB-Waxetr (30m×0.32mm×0.50μm)
[0035] Injection port temperature: 200°C
[0036] Injection volume: 1000μL
[0037] Split ratio: 50:1
[0038] Carrier gas: helium (constant flow 2.0mL / min)
[0039] Headspace conditions: Equilibrium temperature 70°C, quantitative loop temperature 90°C, transfer line temperature 130°C, equilibration time 30min.
[0040] Column temperature: keep at 40°C for 1min, raise the temperature to 100°C at 10°C / min, then raise the temperature to 220°C at 30°C / min, and keep for 3min.
[0041] Detector: mass spectrometer detector, ion source temperature 230°C, transfer line temperature 250°C.
[0042] SIM mode (127, 142): m / z 127 is used as an auxiliary qualifier ion, and m / z 142 is used as a qu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com