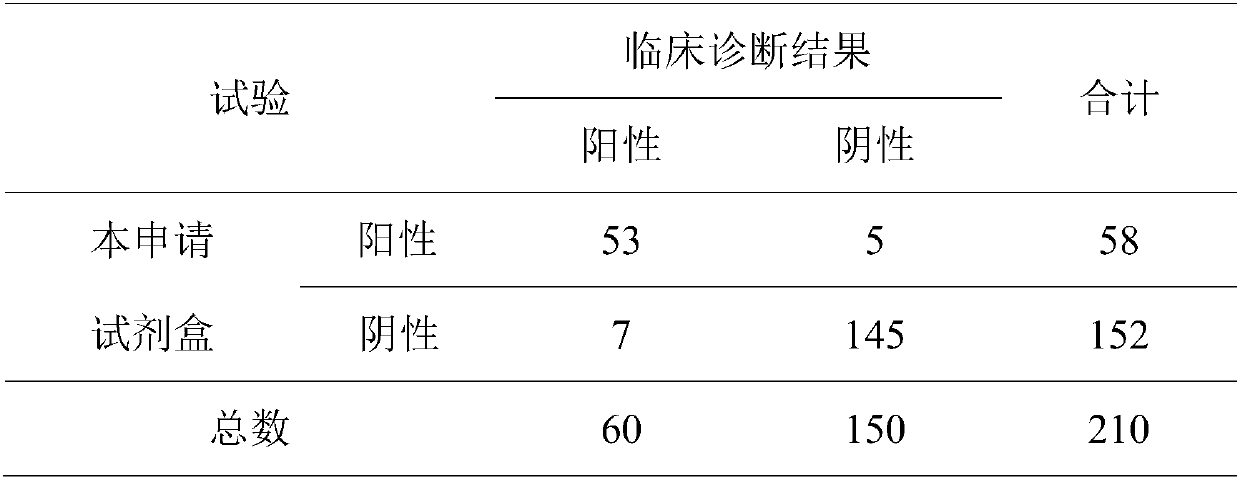
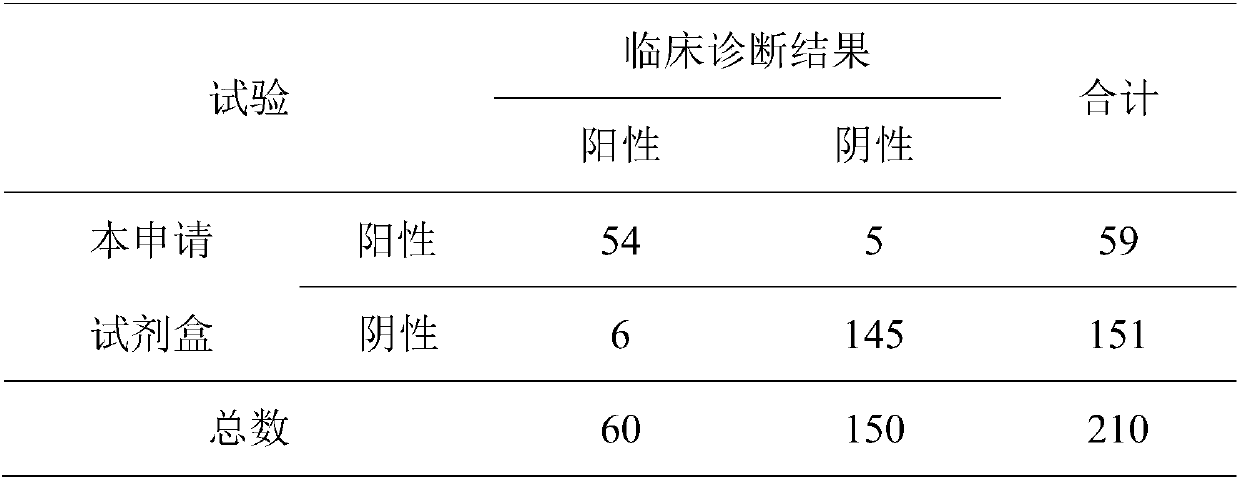
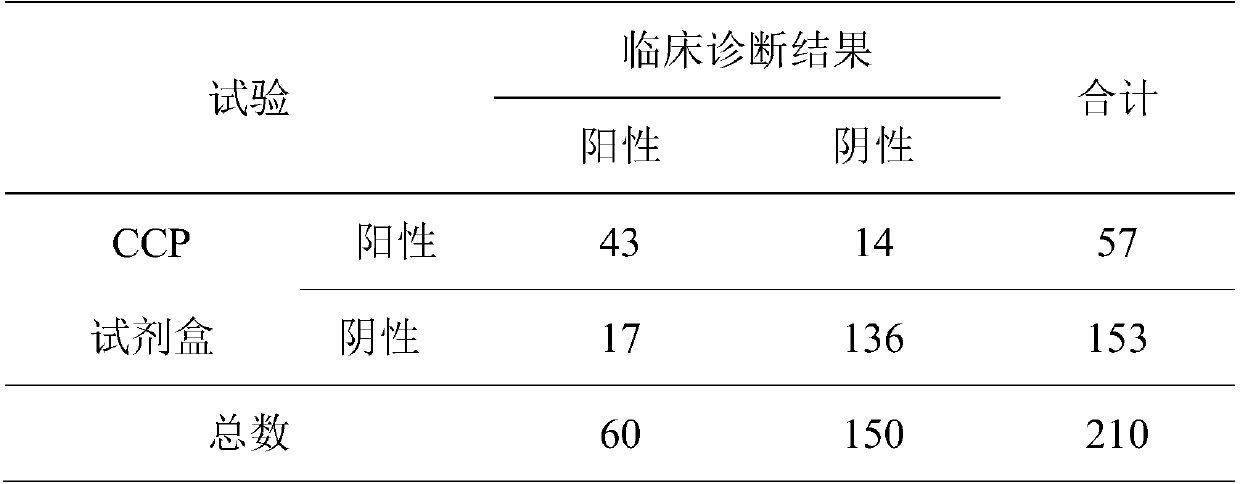
Composite detection antigen and application thereof
A technology for compound detection and combination of antigens, applied to detection kits and their application in the detection of rheumatoid arthritis And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: the assembly of kit
[0068] 1. Main reagents:
[0069] (1) Coating buffer: 0.01M-0.5M pH9.0-pH9.6 carbonate buffer, 0.01%-0.5% ProClin300, pH 6.9-9.4;
[0070] (2) Blocking solution: 0.5-10% BSA, 0.01M-0.5M PBS buffer, 150mM NaCl, 0.01%-0.5% ProClin300, pH 6.9-9.4;
[0071] (3) Sample diluent: 0.5-10% BSA, 0.01M-0.5M Tri-HCL buffer, 0.01%-0.5% ProClin300, pH 6.9-9.4;
[0072] (4) Diluent of negative and positive reference substances: 0.5-10% BSA, 0.01M-0.5M PBS buffer, 0.01%-0.5% ProClin300, pH 6.9-9.4;
[0073] (5) Washing solution: 0.01%-0.2% Tween-20, 0.01M-0.5M PBS buffer, 0.01%-0.5% ProClin300, pH 6.9-9.4;
[0074] (6) Enzyme-labeled antibody diluent: 0.5-10% BSA, 0.01M-0.5M PBS buffer, 0.01%-0.5% ProClin300, pH 6.9-9.4;
[0075] (7) Chromogenic buffer: 0.01M-1M phosphate-citrate buffer, pH 3.0-5.0;
[0076] (8) Chromogenic solution: TMB;
[0077] (9) Termination solution: sulfuric acid.
[0078] 2. Preparation of reaction plate working soluti...
Embodiment 2
[0085] Embodiment 2: the assembly of kit
[0086] 1. Main reagents:
[0087] (1) Coating buffer: 0.01M-0.5M PBS buffer, 0.01%-0.5% ProClin300, pH6.9-9.4;
[0088] (2) Blocking solution: 0.5-10% BSA, 0.01M-0.5M PBS buffer, 0.01%-0.5% ProClin300, pH6.9-9.4;
[0089] (3) Sample diluent: 0.5-10% BSA, 0.01M-1M Tri-HCL buffer, 0.01%-0.5% ProClin300, pH 6.9-9.4;
[0090] (4) Diluent of negative and positive reference substances: 0.5-10% BSA, 0.01M-0.5M PBS buffer, 0.01%-0.5% ProClin300, pH 6.9-9.4;
[0091] (5) Washing solution: 0.01%-0.2% Tween-20, 0.01M-0.5M PBS buffer, 0.01%-0.5% ProClin300, pH 6.9-9.4;
[0092](6) Enzyme-labeled antibody diluent: 0.5-10% BSA, 0.01M-0.5M PBS buffer, 0.01%-0.5% ProClin300, pH 6.9-9.4;
[0093] (7) Chromogenic buffer: 0.01M-1M phosphate-citrate buffer, pH 3.0-5.0;
[0094] (8) Chromogenic solution: TMB;
[0095] (9) Termination solution: sulfuric acid.
[0096] 2. Preparation of reaction plate working solution
[0097] Two types of post-tran...
Embodiment 3
[0103] Embodiment 3: the usage method of kit
[0104] (1) Pretreatment: place each component of the kit at room temperature to equilibrate for 0.5-1h, and dilute the sample 1:50 with the sample diluent;
[0105] (2) Adding samples: Add 100 μl / well of the diluted sample to be tested and negative and positive controls to the corresponding wells of the microplate, and seal the plate with a sealing film;
[0106] (3) Incubation: place the loaded microtiter plate on a shaker at 200rpm-400rpm and incubate at room temperature for 30-60min;
[0107] (4) Washing: the incubated ELISA plate was discarded and patted dry, and washed 4-6 times with washing liquid;
[0108] (5) Add enzyme-labeled antibody: add enzyme-labeled antibody to the enzyme-labeled plate at 100 μl / well, and seal the plate with a sealing film;
[0109] (6) Incubation: Place the added ELISA plate on a 200rpm-400rpm shaker and incubate at room temperature for 30-60min;
[0110] (7) Washing: the incubated ELISA plate w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com