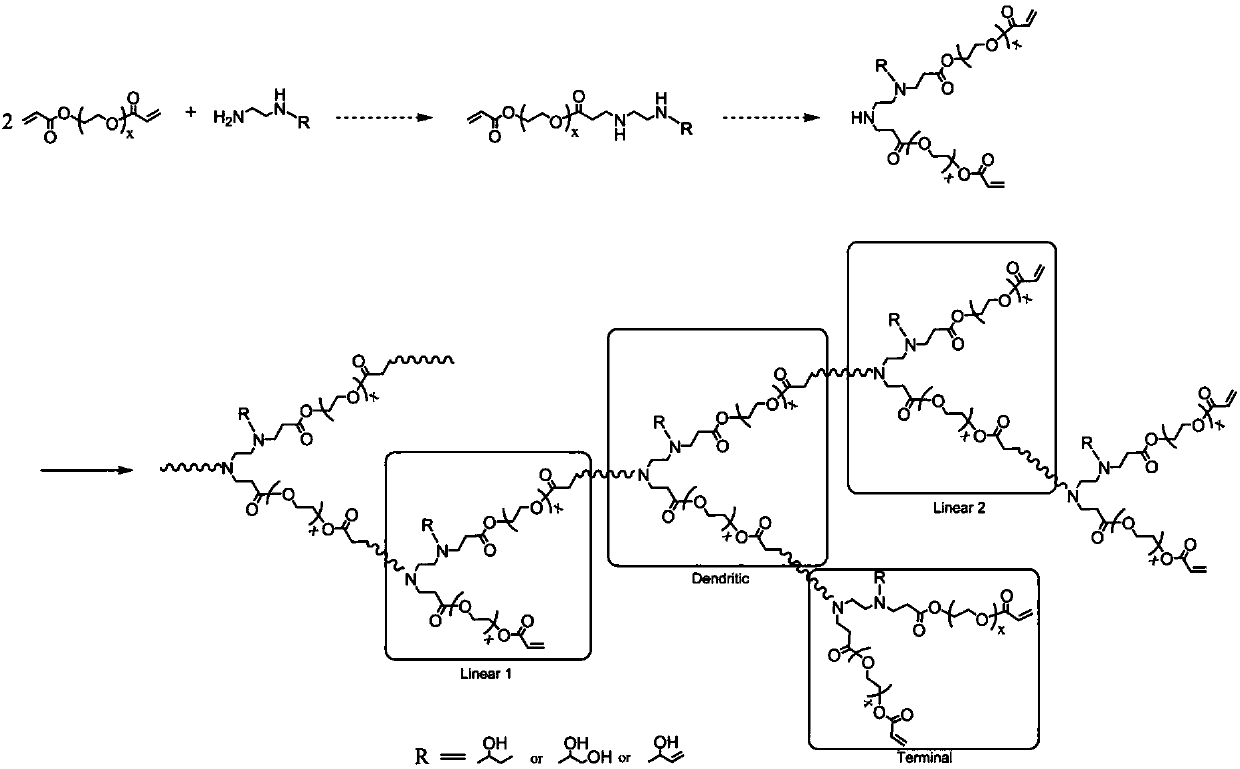
Hyperbranched poly (ester-amine) containing functional groups on both surface and interior and preparation method of hyperbranched poly (ester-amine)
A technology of hyperbranched polymer and functional group, which is applied in the field of hyperbranched polymer and its preparation, can solve the problem that the internal cavity of hyperbranched polymer cannot be fully utilized, and achieve the effect of simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
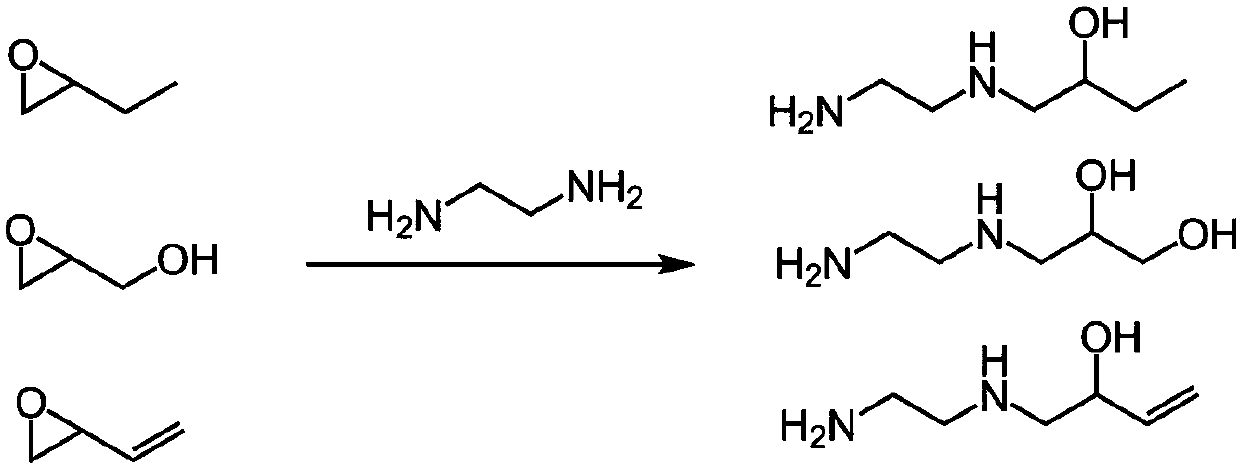
[0022] Into the 500 mL flask was added 208.36 g (3.467 mol) of ethylenediamine. Then 5.000g (0.0693mol) of butylene oxide was dissolved in 50mL of methanol, and was added dropwise to the reaction system under a nitrogen atmosphere, and reacted for 12 hours after the dropwise addition; after the reaction, the reaction solution was rotary evaporated to remove excess ethylene glycol amine to give the product 1-((2-aminoethyl)amino)-2-butanol (5.032 g, yield 54.88%) as a light yellow liquid.
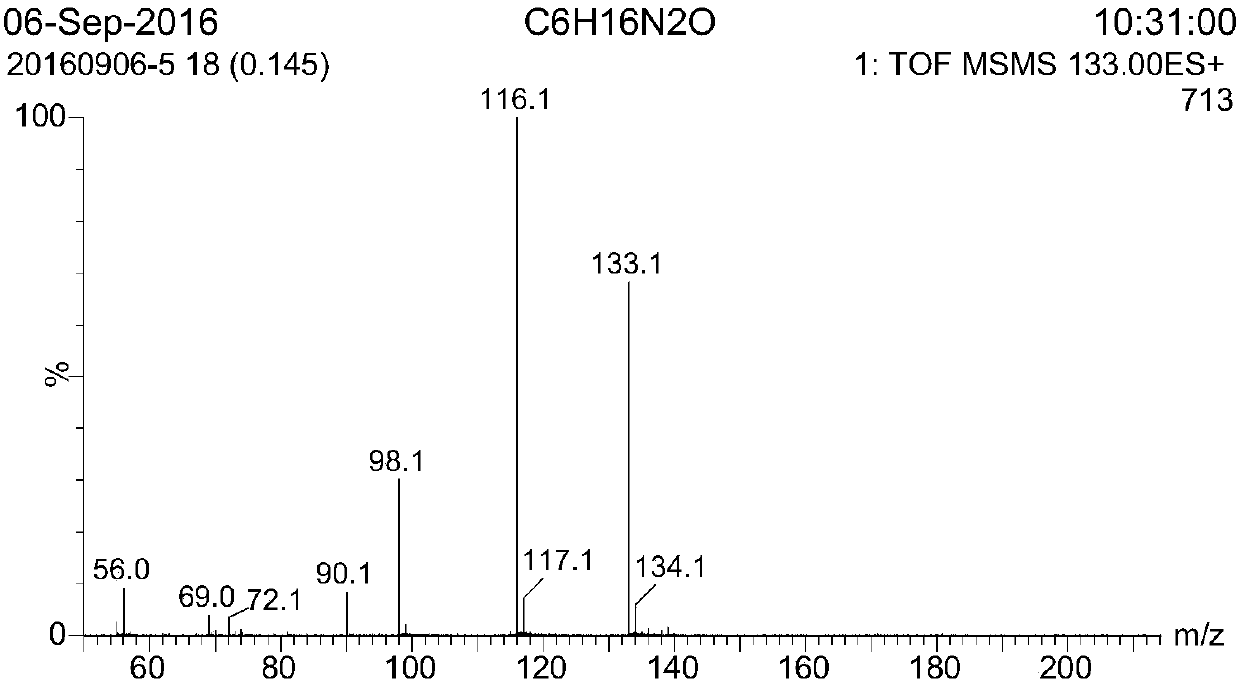
[0023] The mass spectrogram of gained 1-((2-aminoethyl) amino)-2-butanol in embodiment case 1 is as attached figure 2 As shown, the theoretical molecular weight of 1-((2-aminoethyl)amino)-2-butanol is 132.2, and the LC-MS test result is m / z 133.1 ((M+H) + / 1), the test results are consistent with the theoretical calculation values.
Embodiment example 2
[0025] Into the 500 mL flask was added 208.36 g (3.467 mol) of ethylenediamine. Then 5.137g (0.0693mol) of glycidol was dissolved in 50mL of methanol, and was added dropwise to the flask under a nitrogen atmosphere, and stirred for 12 hours after the dropwise addition; after the reaction, the reaction solution was rotary evaporated to remove excess ethylenediamine to obtain The pale yellow liquid product 3-((2-aminoethyl))-1,2-propanediol (5.866 g, 63.08% yield).
Embodiment example 3
[0027] Into the 500 mL flask was added 208.36 g (3.467 mol) of ethylenediamine. Then 4.931g (0.0693mol) of 3,4-epoxy-1-butene was dissolved in 50mL of methanol, and was added dropwise to the flask under a nitrogen atmosphere, and stirred for 12 hours after the addition; after the reaction, the reaction solution was rotated Excess ethylenediamine was removed by evaporation to give the product 1-((2-aminoethyl)amino)-2-hydroxy-3-butene as a light yellow liquid (4.719 g, 52.31% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com