Method for establishing HPLC fingerprint of Yinzhihuang granule, and standard fingerprint obtained therethrough
A fingerprint and standard fingerprint technology, applied in the field of traditional Chinese medicine preparation analysis, to achieve good baseline separation, accurate evaluation, and good reproducibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Establishment of HPLC Standard Fingerprint of Yinzhihuang Granules
[0022] 1 Instruments and reagents
[0023] 1.1 Instrument
[0024] Agilent 1100 high performance liquid chromatography (USA): DAD detector, quaternary low-pressure gradient pump, AgilentOpen Lab chromatography workstation.
[0025] 1.2 Reagent
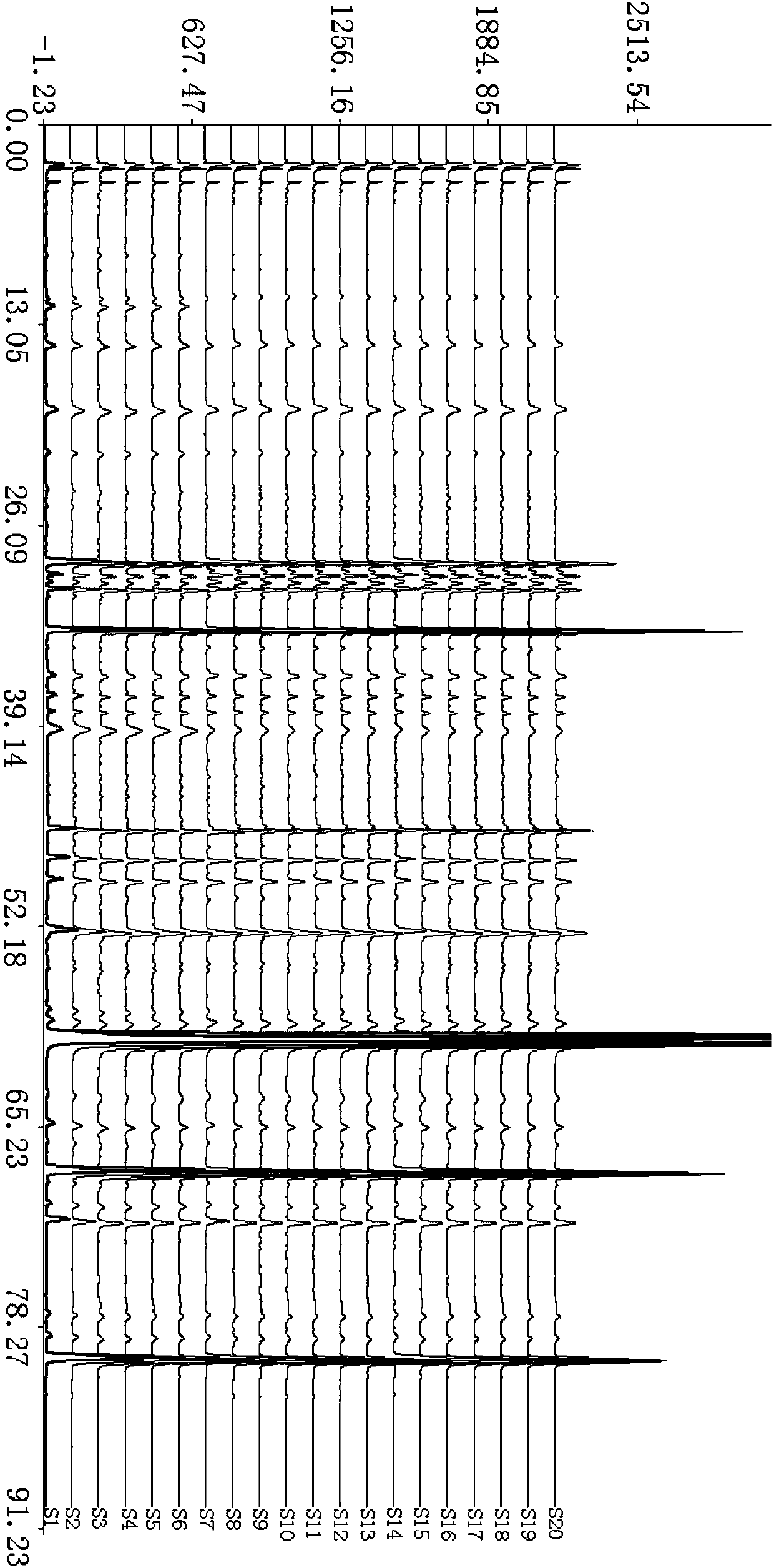
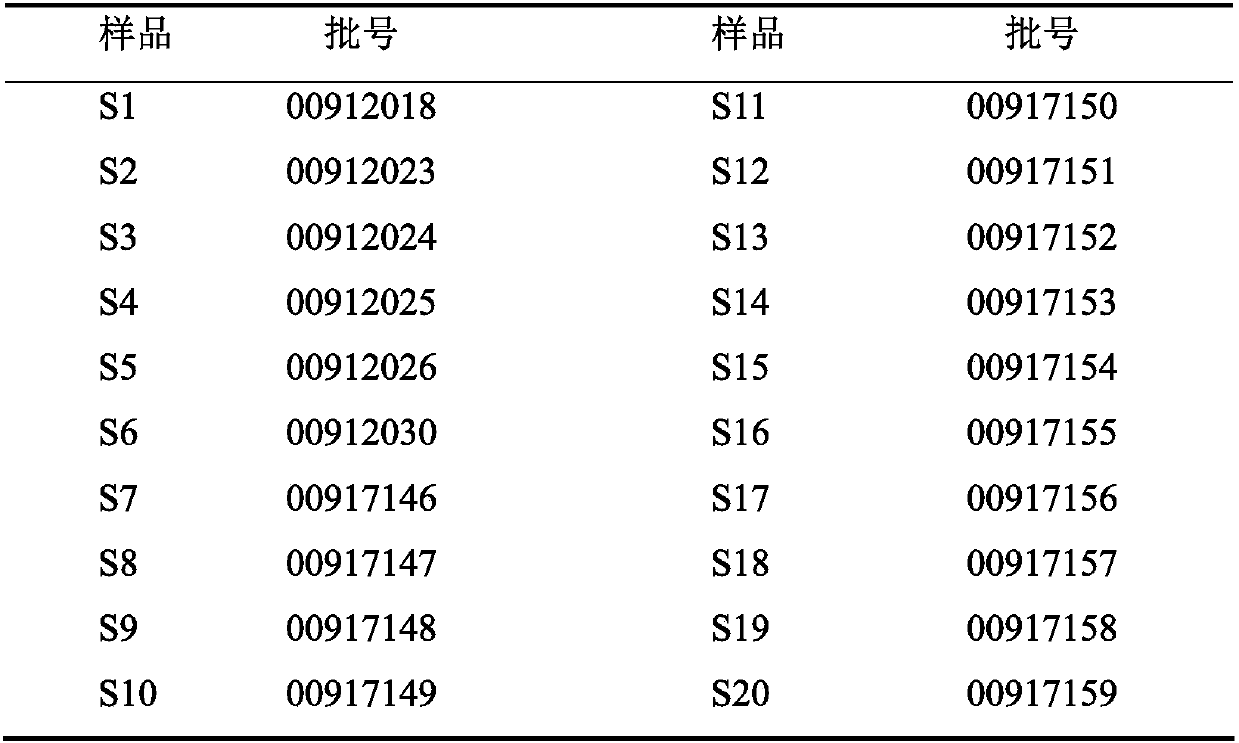
[0026] Yinzhihuang Granules were provided by Lunan Houpu Pharmaceutical Co., Ltd., as shown in Table 1; acetonitrile was chromatographically pure, water was double distilled water, and the rest of the reagents were analytically pure.
[0027] Table 1 Batch numbers of test samples of Yinzhihuang Granules
[0028]
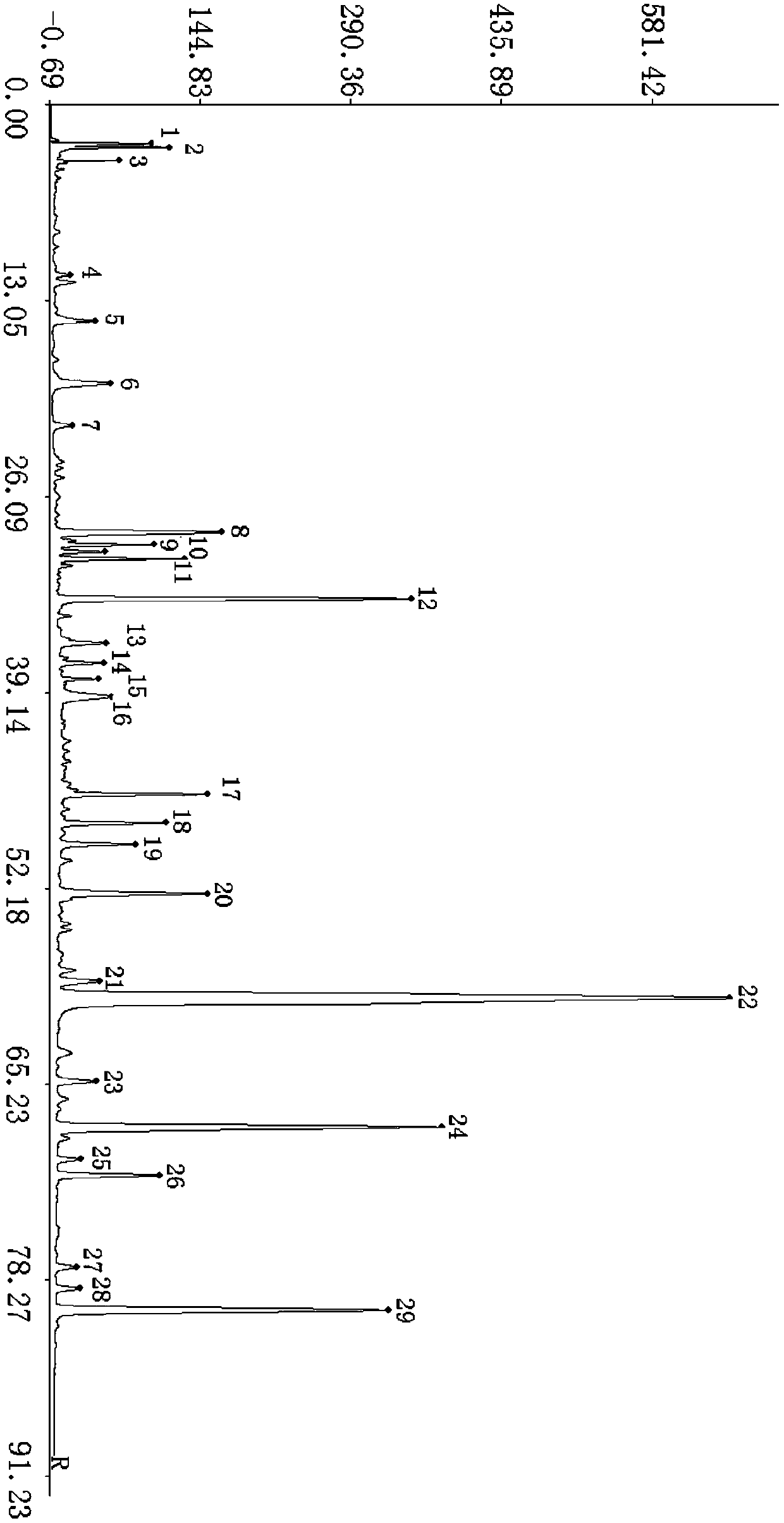
[0029] 2 Methods and results
[0030] 2.1 Chromatographic conditions: Chromatographic column: Tianjin Turner Kromasil C 18 (4.6x250mm, 5um) column; mobile phase: acetonitrile is used as mobile phase A, and 0.1% phosphoric acid aqueous solution is used as mobile phase B in volume percentage, and the gradient elution is carried out acc...
Embodiment 2
[0054] Example 2 Establishment of HPLC Standard Fingerprint of Yinzhihuang Granules
[0055] 1 Instruments and reagents
[0056] 1.1 Instrument
[0057] Agilent 1100 high performance liquid chromatography (USA): DAD detector, quaternary low-pressure gradient pump, AgilentOpen Lab chromatography workstation.
[0058] 1.2 Reagent
[0059] Yinzhihuang Granules were provided by Lunan Houpu Pharmaceutical Co., Ltd., as shown in Table 1; acetonitrile was chromatographically pure, water was double distilled water, and the rest of the reagents were analytically pure.
[0060] Table 1 Batch numbers of test samples of Yinzhihuang Granules
[0061]
[0062] 2 Methods and results
[0063] 2.1 Chromatographic conditions: Chromatographic column: Tianjin Turner Kromasil C 18 (4.6x250mm, 5um) column; mobile phase: acetonitrile is used as mobile phase A, and 0.05% phosphoric acid aqueous solution is used as mobile phase B, and the gradient elution is carried out as follows:
[0064] ...
Embodiment 3
[0069] Example 3 Establishment of HPLC Standard Fingerprint of Yinzhihuang Granules
[0070] 1 Instruments and reagents
[0071] 1.1 Instrument
[0072] Agilent 1100 high performance liquid chromatography (USA): DAD detector, quaternary low-pressure gradient pump, AgilentOpen Lab chromatography workstation.
[0073] 1.2 Reagent
[0074] Yinzhihuang Granules were provided by Lunan Houpu Pharmaceutical Co., Ltd., as shown in Table 1; acetonitrile was chromatographically pure, water was double distilled water, and the rest of the reagents were analytically pure.
[0075] Table 1 Batch numbers of test samples of Yinzhihuang Granules
[0076]
[0077] 2 Methods and results
[0078] 2.1 Chromatographic conditions: Chromatographic column: Tianjin Turner Kromasil C 18 (4.6x250mm, 5um) column; mobile phase: acetonitrile is used as mobile phase A, and 0.2% phosphoric acid aqueous solution is used as mobile phase B in volume percentage, and the gradient elution is carried out in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com