Preparation method of ciprofloxacin hydrochloride tablets
A technology of ciprofloxacin hydrochloride and tablet compression, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas. It can solve the problems of large differences in dissolution rate and stickiness, and achieve different Small size, smooth drug release, good quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
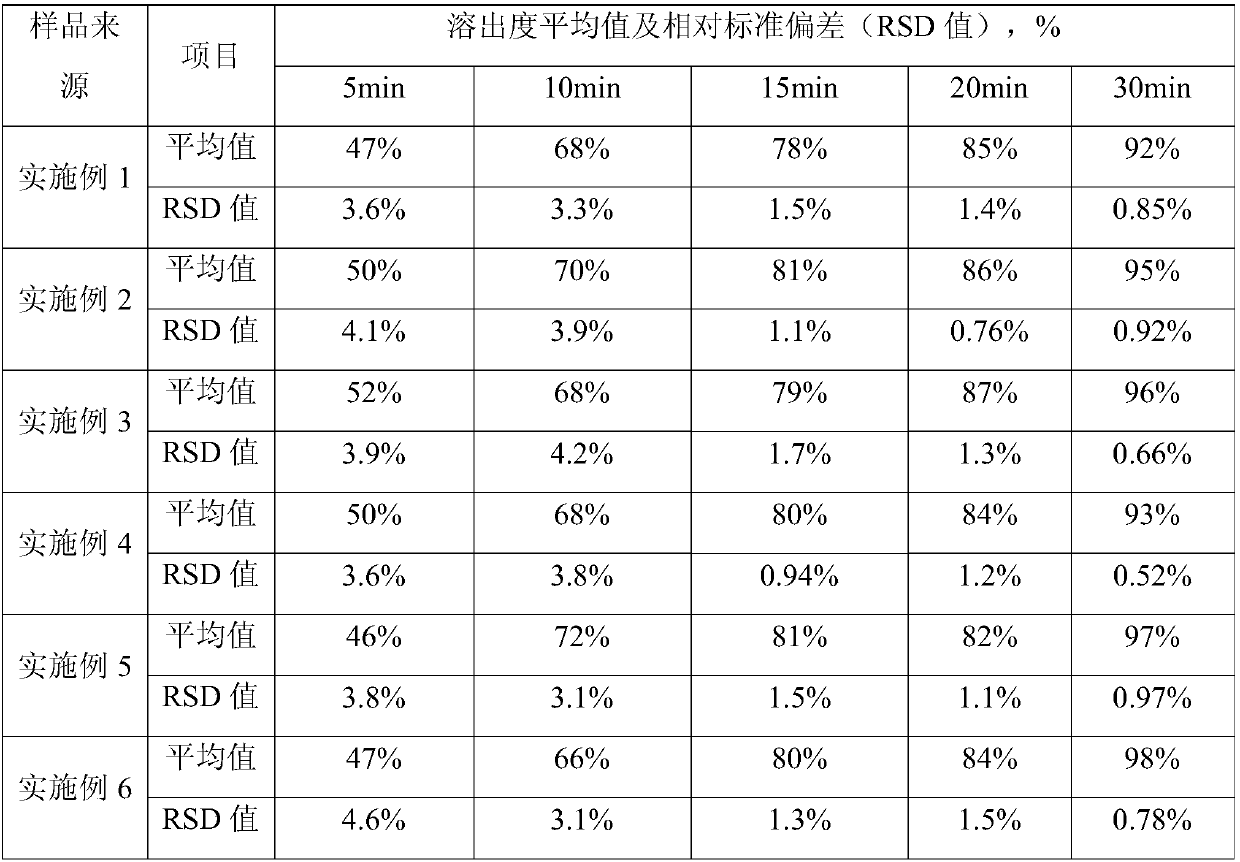
Examples
Embodiment 1
[0024] Prescription composition:
[0025] Ciprofloxacin hydrochloride 588g, lactose 40g, starch 60g, crospovidone 7g, magnesium stearate 3g; the lactose is Foremost315 / 316 type.
[0026] The preparation method of described ciprofloxacin hydrochloride tablet is as follows:
[0027] (1) Ciprofloxacin hydrochloride is passed through an 80-mesh sieve, and lactose, starch, crospovidone and magnesium stearate are passed through an 80-mesh sieve respectively;
[0028] (2) take by weighing ciprofloxacin hydrochloride, lactose, starch, crospovidone, magnesium stearate;
[0029] (3) Add ciprofloxacin hydrochloride, lactose, starch, and crospovidone into the mixer, and mix for 25 minutes at a speed of 8 rpm;
[0030] (4) Magnesium stearate was added to the mixer, and mixed for 5 minutes at a rotating speed of 8 rpm;
[0031] (5) The mixture is directly compressed into tablets, and the average hardness of the tablets is 60N.
Embodiment 2
[0033] Prescription composition:
[0034] Ciprofloxacin hydrochloride 588g, starch 60g, lactose 40g, microcrystalline cellulose 50g, crospovidone 12g, magnesium stearate 4g; starch is compressible starch, lactose is Foremost 315 / 316 type, microcrystalline Cellulose is PH102 type.
[0035] The preparation method of described ciprofloxacin hydrochloride tablet is as follows:
[0036] (1) Ciprofloxacin hydrochloride is passed through a 100-mesh sieve, and starch, lactose, microcrystalline cellulose, crospovidone, and magnesium stearate are passed through a 100-mesh sieve respectively;
[0037] (2) Take by weighing ciprofloxacin hydrochloride, starch, lactose, microcrystalline cellulose, crospovidone, magnesium stearate;
[0038] (3) Add ciprofloxacin hydrochloride, starch, lactose, microcrystalline cellulose, and crospovidone into the mixer, and mix for 25 minutes at a speed of 8 rpm;
[0039] (4) Magnesium stearate was added to the mixer, and mixed for 5 minutes at a rotating...
Embodiment 3
[0042] Prescription composition:
[0043] Ciprofloxacin hydrochloride 588g, starch 100g, lactose 50g, microcrystalline cellulose 50g, crospovidone 15g, magnesium stearate 5g; starch is compressible starch, lactose is Foremost 315 / 316 type, microcrystalline Cellulose is PH102 type.
[0044] The preparation method of described ciprofloxacin hydrochloride tablet is as follows:
[0045] (1) Ciprofloxacin hydrochloride is passed through a 120-mesh sieve, and starch, lactose, microcrystalline cellulose, crospovidone, and magnesium stearate are passed through a 120-mesh sieve respectively;
[0046] (2) Take by weighing ciprofloxacin hydrochloride, starch, lactose, microcrystalline cellulose, crospovidone, magnesium stearate;
[0047] (3) Add ciprofloxacin hydrochloride, starch, lactose, microcrystalline cellulose, and crospovidone into the mixer, and mix for 25 minutes at a speed of 8 rpm;
[0048] (4) Magnesium stearate was added to the mixer, and mixed for 5 minutes at a rotatin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average hardness | aaaaa | aaaaa |
| Average hardness | aaaaa | aaaaa |
| Average hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com