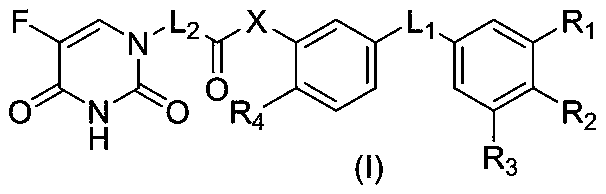
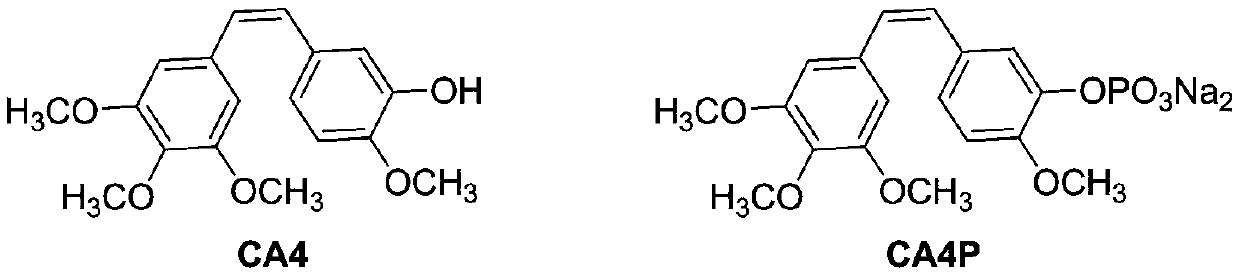
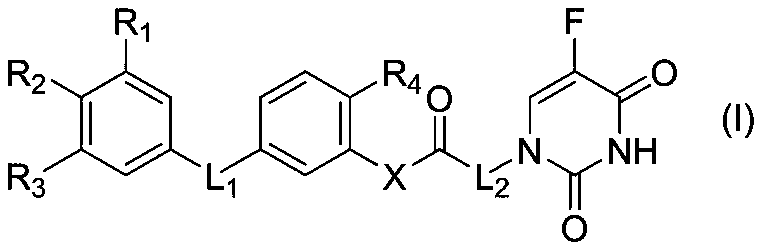
Preparation and application of a 5-fluorouracil substituted carboxylic acid derivative
A technology for fluorouracil carboxylic acid and derivatives, which is applied in the field of medicinal chemistry to achieve the effects of reducing toxic side effects, improving oral bioavailability, and having a broad anti-tumor spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Preparation of 3'-amino-3,4,4',5-tetramethoxystilbene (Ⅱa)
[0035]
[0036] Step 1: Synthesis of 3'-nitro-3,4,4',5-tetramethoxystilbene
[0037] Under the protection of nitrogen, suspend and dissolve 3,4,5-trimethoxybenzyl bromidetriphenylphosphine salt (4.42g, 8.5mmol) in anhydrous tetrahydrofuran (50mL), cool down to -20°C, and slowly add dropwise under stirring n-Butyllithium solution (8mL, 2.5M), gradually dissolved after adding, and continued to stir for 3h, dissolved 3-nitro-4-methoxybenzaldehyde (1.5g, 8.3mmol) in 10mL of anhydrous tetrahydrofuran, Add dropwise to the reaction solution through the dropping funnel, after the addition is complete, react for 5 hours and then rise to room temperature, and stir the reaction overnight. TLC detected that the reaction was complete, and 50 mL was added to quench the reaction, and the liquid was separated. Re-extract with 50mL ethyl acetate, combine the organic phases, continue to wash with water for 2-3 ti...
Embodiment 2
[0040] Example 2 Synthesis of 3'-amino-3,4,4',5-tetramethoxydiphenylethane (Ⅱb)
[0041]
[0042] Dissolve the 3'-nitro-3,4,4',5-tetramethoxystilbene (1.4g, 4.0mmol) prepared in Step 1 of Example 1 in 30mL ethyl acetate, add 10% Palladium carbon catalyst (200mg), replaced with hydrogen three times, hydrogenated at room temperature for 4h, TLC detected the reaction was complete, filtered, spin-dried, and purified by column chromatography to obtain IIb (1g, 78.0%). 1 H NMR (500MHz, CDCl 3 )δ6.76(d, J=5Hz 2H), 6.65(d, J=5Hz, 1H), 6.41(s, 2H), 3.87(s, 3H), 3.85(s, 9H), 2.82(s, 4H ).
Embodiment 3
[0043] Example 3 3,4,5-trimethoxy-3'-amino-4'-ethoxystilbene (Ⅱc)
[0044]
[0045] Step 1: Preparation of 3,4,5-trimethoxyphenyl-3'-nitro-4'-ethoxystilbene
[0046] Under the protection of argon, suspend trimethoxyphenylmethylenetriphenylphosphine bromide (15g, 28.7mmol) in anhydrous tetrahydrofuran (300mL), cool to about -15°C, drop n-butyl lithium cyclohexyl Alkane solution (1.6mol / L, 22mL), after adding, react for 1 hour, dissolve 4-ethoxy-3-nitrobenzaldehyde (5.7g, 29mmol) in anhydrous tetrahydrofuran (24mL), and drop The funnel was slowly added dropwise into the above reaction solution, after the addition was completed, it was stirred for 1 h and then raised to room temperature, and stirred overnight, TLC detection showed that the reaction was complete, adding water to quench the reaction, separating the organic layer, removing 3 / 4 of the solvent, and adding 4 of the remaining mother liquor. The mixture was doubled with absolute ethanol, cooled in an ice bath for cry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com