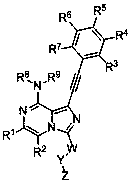
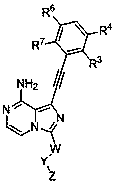
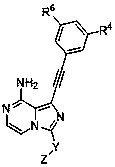
Heterocyclic compound used as FGFR inhibitor
A compound, heterocyclic group technology, applied in the field of fibroblast growth factor-related receptor FGFR inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] ( S )-1-((3,5-dimethoxyphenyl)ethynyl)-3-(1-acryloylpyrrolidin-3-yl)imidazo[1,5-a]pyrazin-8-amine
[0151]
[0152]
[0153] first step
[0154] ( S )-3-(((3-chloropyrazin-2-yl)methyl)carbamoyl)pyrrolidine-1-carboxylic acid benzyl ester
[0155] Compound (3-chloropyrazin-2-yl)methanamine hydrochloride 1a (3.1 g, 21.7 mmol), O-benzotriazole- N , N , N , N -Tetramethylurea tetrafluoroborate (7.1 g, 22 mmol), diisopropylethylamine (9.3 g, 72 mmol) and dichloromethane (100 mL) were mixed and added in portions at room temperature ( S )-1-((benzyloxy)carbonyl)pyrrolidine-3-carboxylic acid (4.5 g, 18 mmol), stirred at room temperature for 16 hours. The mixture was quenched with 30 mL of water, the organic phase was separated, the aqueous phase was extracted with dichloromethane (50 mL × 2), the combined organic phases were washed with saturated brine (50 mL × 2). The combined organic phases were dried over anhydrous sodium sulfate, filtered to remove the desicca...
Embodiment 2
[0183] 1-((3,5-dimethoxyphenyl)ethynyl)-3-(1-acryloylazetidin-3-yl)imidazo[1,5-a]pyrazin-8-amine
[0184]
[0185]
[0186] first step
[0187] Benzyl 3-(((3-chloropyrazin-2-yl)methyl)carbamoyl)azetidine-1-carboxylate
[0188] Compound (3-chloropyrazin-2-yl)methanamine hydrochloride 1a (0.9 g, 5.0 mmol), N , N -Dimethylformamide (0.02 mL) and dichloromethane (20 mL) were mixed, and 3-(chloroformyl)azetidine-1-carboxylic acid benzyl ester (1.3 g, 5.0 mmol) was added dropwise at room temperature Dichloromethane solution (5.0 mL), stirred at room temperature for 10 min. The mixture was quenched with 30 mL saturated aqueous sodium bicarbonate solution, the organic phase was separated, the aqueous phase was extracted with dichloromethane (30 mL × 2), the combined organic phases were washed with saturated brine (30 mL × 2). The combined organic phases were dried over anhydrous sodium sulfate, filtered to remove the desiccant, and the solvent was removed under reduced pres...
Embodiment 3
[0216] ( R )-1-((3,5-dimethoxyphenyl)ethynyl)-3-(1-acryloylpyrrolidin-3-yl)imidazo[1,5-a]pyrazin-8-amine
[0217]
[0218] Synthetic Example 3 with reference to the operating steps of Example 1, but in the first step with ( R )-1-((Benzyloxy)carbonyl)pyrrolidine-3-carboxylic acid instead of ( S )-1-((benzyloxy)carbonyl)pyrrolidine-3-carboxylic acid.
[0219] MS m / z (ESI): 418 [M+1]
[0220] 1 H NMR (400 MHz, CDCl3) δ 7.24-7.11 (m, 2H), 6.71 (s, 2H), 6.50-6.37 (m,3H), 6. 19-5.84 (bs, 2H), 5.78-5.70 (m , 1H), 4.16-4.07 (m, 2H), 3.96-3.93 (m,1H), 3.80 (s, 6H), 3.80-3.61 (m, 2H), 2.46-2.22 (m, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ic50 | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com