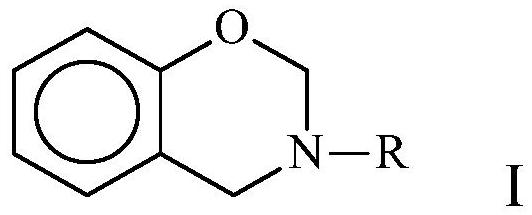
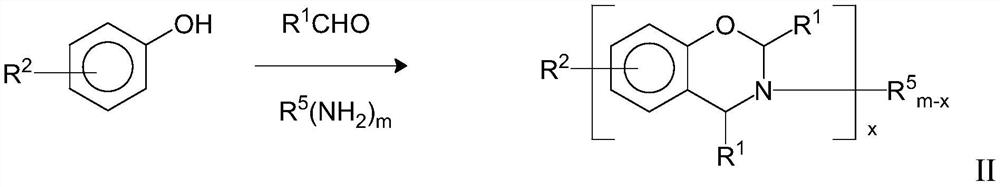
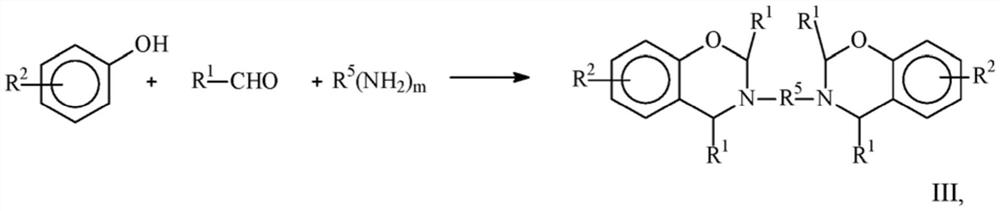
Ammonium Salt Catalyzed Polymerization of Benzoxazines
A kind of technology of benzoxazine and ammonium salt, applied in the field of preparing polybenzoxazine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0166] All materials were used as received from chemical suppliers without further purification.
[0167] Table 1: Indexed Materials - Abbreviations
[0168]
[0169]
[0170] testing method
[0171] Differential Scanning Calorimetry (DSC Analysis)
[0172] Samples were prepared by melting the appropriate amount of benzoxazine and mixing the required amount of amine salt. Unless otherwise specified, mixtures were 10 molar equivalents of monofunctional benzoxazine (Ph-a) to 1 molar equivalent of amine salt. For the bifunctional BisA-a benzoxazine, the mixture is 5 molecular equivalents of monomer to 1 equivalent of amine salt, so that the total molar equivalent of benzoxazine functionality to amine salt remains at 10:1.
[0173] The thermal properties of the composition during curing were determined by placing a 4-8 mg aliquot of the composition in an aluminum pan of a Differential Scanning Calorimeter (DSC) and heating at a rate of 10 °C / min from 0°C, 20°C, or ...
preparation example 1-
[0178] Preparation example 1-synthesis of N-benzylmethylamine hydrochloride (BMAC)
[0179] In a glass vial with a magnetic stir bar, N-benzylmethylamine (1.21 g, 0.0100 mol) was dissolved in ethanol (10.0 mL). Concentrated hydrochloric acid (0.60 mL, 0.0105 mol) was added dropwise while stirring the solution vigorously. The reaction was placed in a -10°C freezer overnight. The white precipitate formed was isolated by vacuum filtration and washed with cold ethanol. Recovered mass: 0.98 g (61.8% yield). use 1 H and 13 C NMR confirmed the chemical structure.
preparation example 2-N
[0180] Preparation example 2-N-benzylmethylamine trifluoroacetate (BMATFA)
[0181] In a glass vial with a magnetic stir bar, mix N-benzylmethylamine (1.21 g, 0.0100 moles) and anhydrous trifluoroacetic acid (1.14 g, 0.0100 moles) while stirring vigorously. There was a moderate exotherm upon acid addition and the resulting clear liquid was pale orange. Recovered mass: 2.35 g (quantitative).
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com