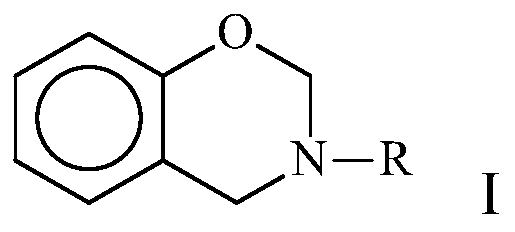
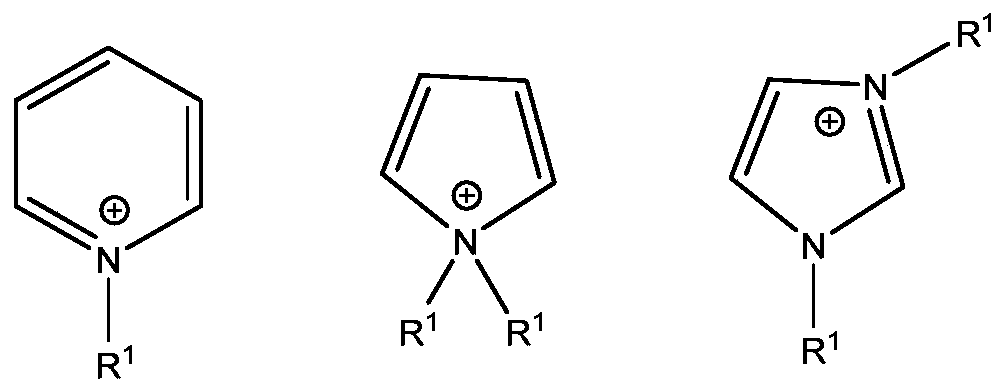
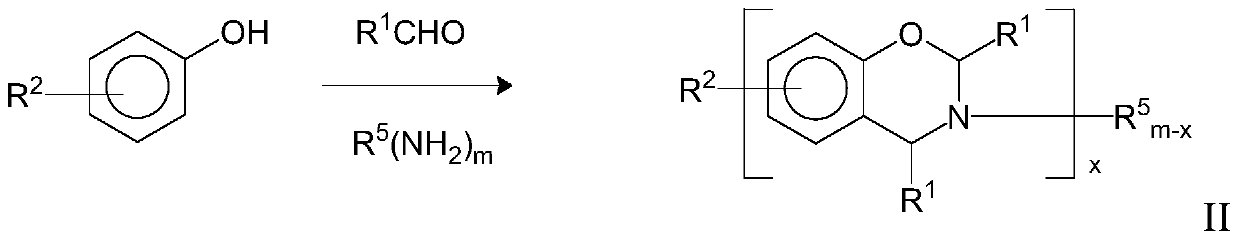
Benzoxazine composition
A technology of benzoxazine and composition, applied in the field of catalysts, to achieve the effect of lowering polymerization temperature and reducing heat release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The preparation of polyiodine can be similar to that of P.Dyson et al., Elemental iodine on imidazolium-based ionic liquids: solution and solid effects (Influence of Elemental Iodine on Imidazolium-BasedIonic Liquids:Solution and Solid-State Effects) , Inorg.Chem. ("Journal of Inorganic Chemistry"), 2015, Volume 54, those procedures described in pages 10504-10512 to complete. DOI10.1021 / acs.inorgchem.5b02021.
[0046] Methods of preparing quaternary ammonium halide salt precursors are known to those skilled in the art. The main method is to react a tertiary amine with the appropriate alkyl halide, haloalkyl ether or epoxide in the presence or absence of solvent. The reaction of tertiary amines with epoxides in aqueous media produces quaternary ammonium hydroxides, which are then neutralized with mineral acids (H-X) to yield quaternary ammonium halides. In this case, complexation with bromine to form the polyhalide ion XBr was determined by the mineral acid H-X used fo...
Embodiment
[0169] Unless specified to the contrary, amounts are given in equivalents (eq). Equivalents are based on moles of reactive groups per mole of reactant molecules. Thus, 2 equivalents of a difunctional reactant would represent one mole of that reactant, and one mole of a trifunctional reactant would represent 3 equivalents of that reactant. The catalyst was treated as a monofunctional catalyst.
[0170] List of abbreviations used :
[0171] BZ-1 Phenol-aniline benzo from Huntsman Advanced Materials
[0172] Oxazine
[0173] BZ-2 Benzoxazines derived from p-cresol, aniline, and paraformaldehyde, via Ishida's U.S. Patent No. 5,
[0174] 543,516 prepared by the method.
[0175] BZ-3 Araldite MT35700 from Huntsman Advanced Materials
[0176] CAT-1 Iodine, CAS#: 7553-56-2 from Sigma-Aldrich, Milwaukee, WI
[0177] (Sigma-Aldrich, Milwaukee, WI).
[0178] CAT-2 1',1"'-Dimethyl-1,1"-dinuclear ferrocenium triiodide, CAS#: 113575-26-1, as in manufacture
[0179] Prepared as de...
preparation example 1
[0199] Following literature procedures, ferrocenium triiodide (CAT-4) salts were synthesized by adding stoichiometric amounts of iodine to neutral ferrocene in a suitable solvent. For example, triiodide is synthesized by adding (dropwise) 3.807 g of iodine dissolved in about 50 mL of pure ethanol to a stirred solution of 1.860 g (0.01 mole) of ferrocene (about 50 mL) also in pure ethanol. Ferrocenium. Before the addition, the solution was warmed slightly (approximately 40°C) to facilitate dissolution of the iodine and ferrocene. The mixture was stirred overnight at room temperature, and the dark blue / black crystallites that precipitated out of ethanol were collected by filtration, washed with additional pure ethanol, and dried under a stream of nitrogen.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com