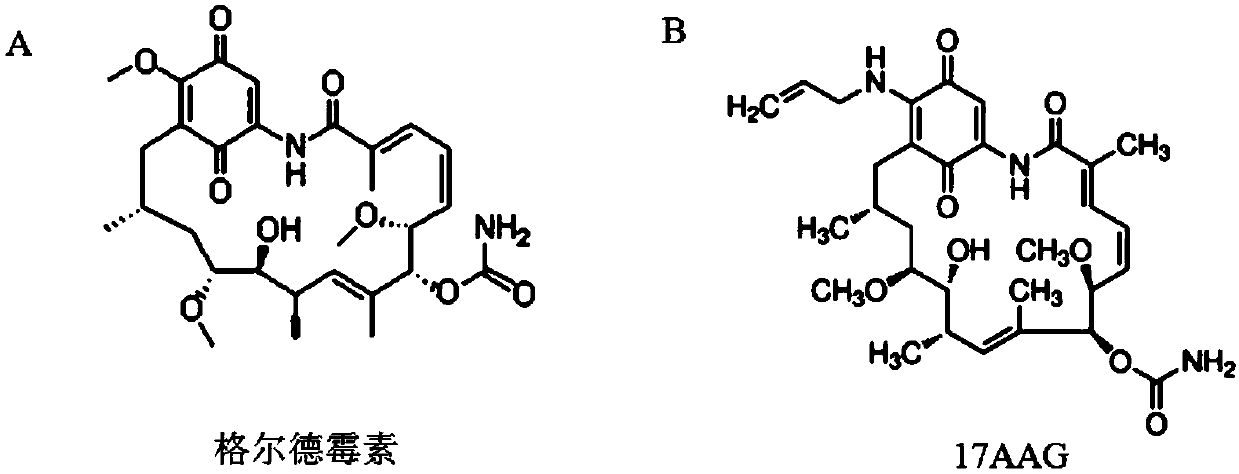
New application of geldanamycin and derivative thereof in treating and inhibiting posterior capsular opacification
A technology of geldanamycin and derivatives, applied in the field of medicine, can solve problems such as unseen cataract
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
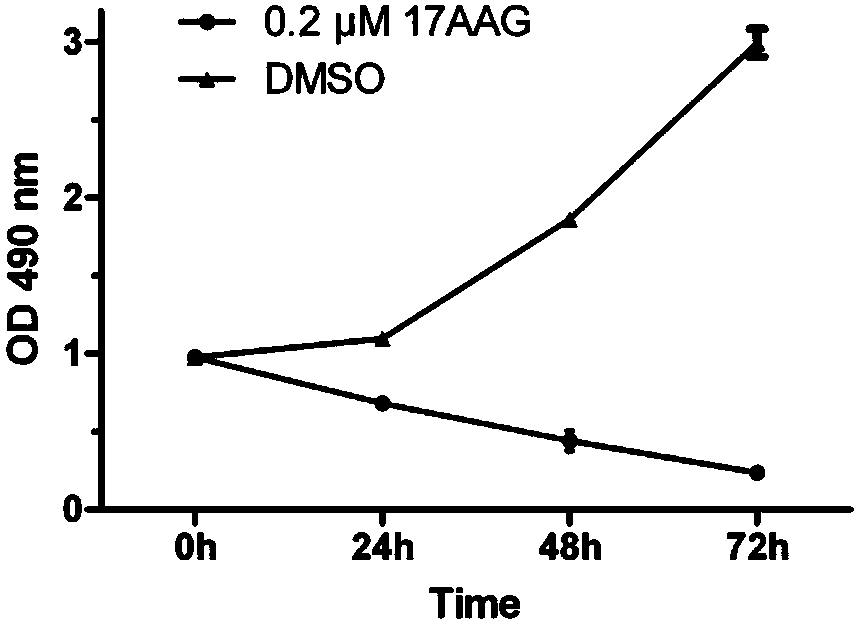
[0036] Inhibitory effect of 17-AAG on cell growth of human lens epithelial cell line HLE-B3.
[0037] Human lens epithelial cell line HLE-B3 cells are commonly used in post-cataract research. We selected HLE-B3 cell line to detect the inhibitory effect of 17AAG on the growth of lens epithelial cells. HLE-B3 cells by 6×10 3 Cells / well were seeded in 96-well culture plates, and the cell culture medium was DMEM containing 10% FBS. After culturing overnight, DMSO and 17AAG were added for treatment. In the 17AAG treatment group, 0.2 μl of 10 mM 17AAG was added to every 10 milliliters of culture medium to make a final concentration of 0.2 μM; in the control group, 0.2 μl of DMSO was added to every 10 milliliters of culture medium. There were 5 repetitions in each group, and they were treated for 0, 24, 48 and 72 hours, respectively. Cell proliferation was detected by MTS kit (Promega Company) combined with a microplate reader, and the detection wavelength was 490 nm.
[0038] T...
Embodiment 2
[0040] (1) 17-AAG inhibits the proliferation and migration of capsular lens epithelial cells cultured in vitro.
[0041] The in vitro mouse or rat lens capsular bag culture model is a commonly used in vitro model for studying post-cataract. We took Wistar rats (7-8 weeks, male or female, 200-250 g) as experimental animals, and after isoflurane inhalation anesthesia, the eyeballs of the rats were taken out, washed 3 times with PBS solution containing 5% penicillin and streptomycin, Remove the lens. Cool the autoclaved 2% cryogenic agar to 37 °C and pour into sterile molds. Place the prepared lens in the center of the agarose and suck out the excess agar to the equator of the lens. Cool the mold to room temperature until the agar solidifies. The anterior capsule of the lens was torn open with capsulorhexis forceps, and lactated Ringer's solution was injected into the capsule bag for water separation, so that the lens cortex and capsule were completely separated and protruded ...
Embodiment 3
[0052] Validation test of 17AAG promoting recovery of opaque lens capsular bag.
[0053] The lens capsule was cultured in vitro with DMEM containing 10% fetal bovine serum. On the third day, lens epithelial cells completely covered the lens capsule. The above crystal capsules were randomly divided into two groups: 17AAG treatment group and DMSO control group, with 3 crystal capsules in each group, and the liquid was changed every 7 days. The final concentration of 17AAG was 0.4 μmol / L, that is, 0.4 μl of 10 mM 17AAG was added to every 10 ml of culture medium. In the control group, 0.4 μl of DMSO was added to every 10 ml of culture medium. After adding the medicine, observe through an inverted microscope every day, and take pictures and record it for a total of 31 days. The result is as Figure 8 As shown, the lens capsular bag was cultured in vitro for 3 days, and the lens epithelial cells completely covered the posterior lens capsule, and then one group was added with 17AA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transparency | aaaaa | aaaaa |
| transparency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com