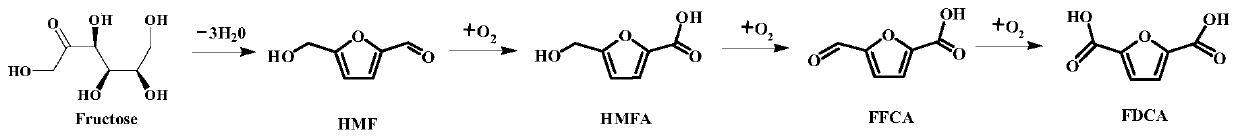
One-pot one-step method for preparing 2,5-furandicarboxylic acid from fructose
A technology of furandicarboxylic acid and fructose, which is applied in the field of bio-based polymer monomer preparation, can solve the problems of increased preparation cost, long separation time, complicated operation, etc., so as to save separation cost and time, reduce raw material loss, and improve economical efficiency. effect of benefit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
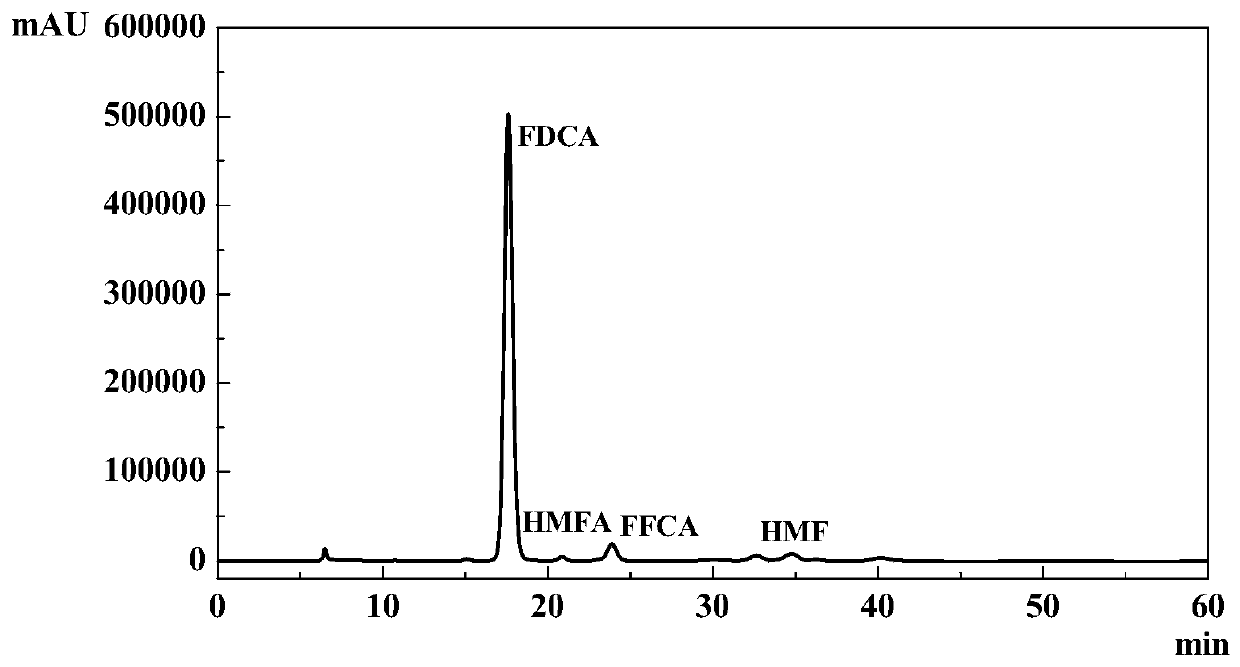
[0020] 1g of ionic liquid [Bmim]Cl and raw material fructose with a mass ratio of 1.80:100, 0.01g of Amberlyst-15, Nafion-NR50, CrCl 3 ·6H 2 O, FeCl 3 ·6H 2 O or AlCl 3 , and 0.01g Fe 0.6 Zr 0.4 o 2Put the catalyst into a 50ml autoclave with PTFE liner, seal the autoclave well, wash the autoclave with oxygen for three times, then react the autoclave at 160°C for 24h under 2Mpa oxygen, then quickly cool it to room temperature, and end the reaction . The products were qualitatively and quantitatively analyzed with Shimazu LC-20A. The influence of different dehydration catalysts on the reaction is detailed in No. 1-5 in Table 1.
[0021] The influence of table one embodiment 1-5 different dehydration catalysts on reaction
[0022]
[0023] 2. Effect of different oxidation catalysts on the reaction
Embodiment 6-14
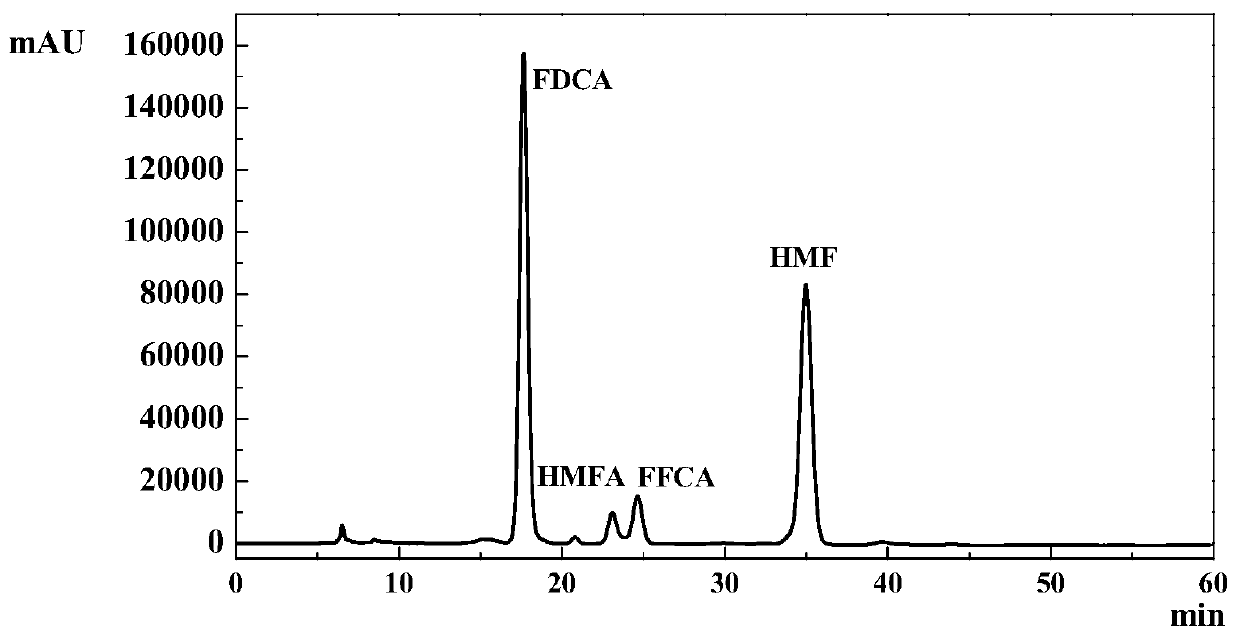
[0025] 1g of ionic liquid [Bmim]Cl and raw material fructose with a mass ratio of 1.80:100, 0.01g of Amberlyst-15 solid acid catalyst, and 0.01g of CeO 2 , Fe 2 o 3 , ZrO 2 , Ce 0.5 Zr 0.5 o 2 , Ce 0.5 Fe 0.5 o 2 , Fe 0.5 Zr 0.5 o 2 , Fe 0.2 Zr 0.8 o 2 , Fe 0.8 Zr 0.2 o 2 or Ce 0.5 Fe 0.15 Zr 0.35 o 2 Put the catalyst into a 50ml autoclave with PTFE liner, seal the autoclave well, wash the autoclave with oxygen for three times, then react the autoclave at 160°C for 24h under 2Mpa oxygen, then quickly cool it to room temperature, and end the reaction . The products were qualitatively and quantitatively analyzed with Shimazu LC-20A. The influence of different dehydration catalysts on the reaction is shown in No. 6-14 in Table 2.
[0026] The influence of table two embodiment 6-14 different oxidation catalysts on reaction
[0027]
[0028] 3. Effect of different ionic liquids on the reaction
Embodiment 15-23
[0030] 1g ionic liquid [Bmim]DMP, [Bmim]Br, [Emim]Cl, [Emim]Br, [Hmim]Cl, [Omim]Cl, [Dmim]Cl, [C 12 mim]Cl and [C 14 mim]Cl and the raw material fructose that its mass ratio is 1.80:100, 0.01gAmberlyst-15 solid acid catalyst, and 0.01gFe 0.6 Zr 0.4 o 2 Add the oxidation catalyst to a 50ml autoclave with PTFE liner, seal the autoclave well, wash the autoclave with oxygen for three times, then react the autoclave at 160°C for 24 hours under 2Mpa oxygen, then quickly cool it to room temperature, and finish reaction. The products were qualitatively and quantitatively analyzed with Shimazu LC-20A. The influence of different dehydration catalysts on the reaction is shown in No. 15-23 in Table 3.
[0031] The influence of table three embodiment 15-23 different ionic liquids on reaction
[0032]
[0033] 4. The influence of different oxidants on the reaction
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com