Beta-caryophyllene derivative as well as preparation method and application thereof
A kind of technology of caryophyllene and derivative, applied in β-caryophyllene derivative and field of preparation and application thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
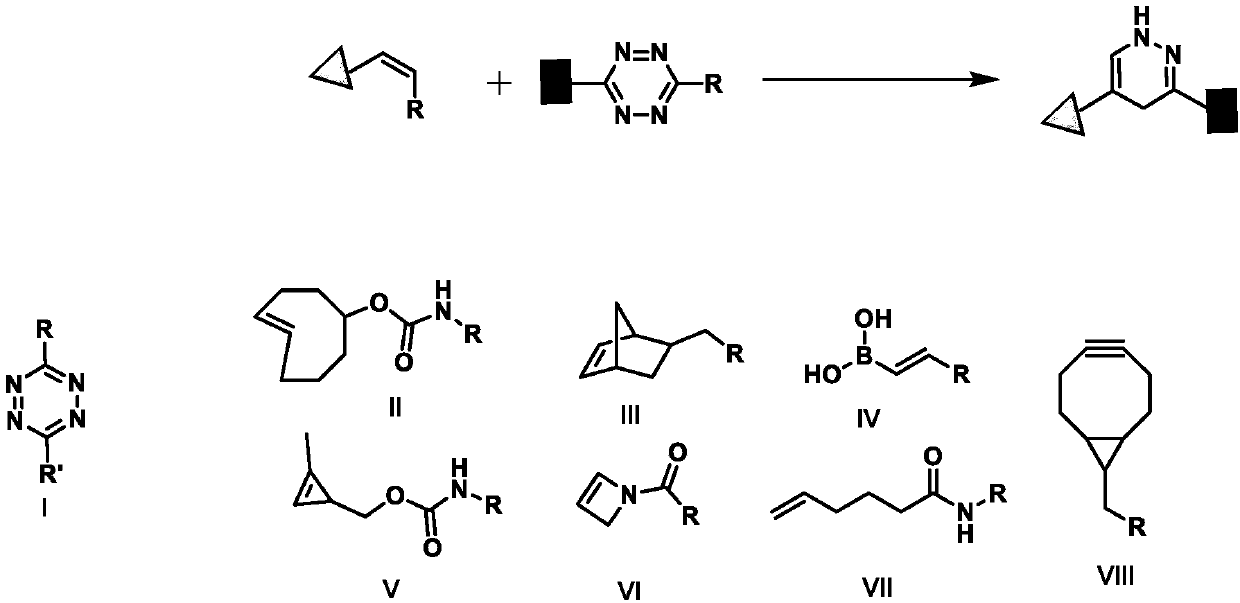
Method used
Image
Examples
Embodiment 1
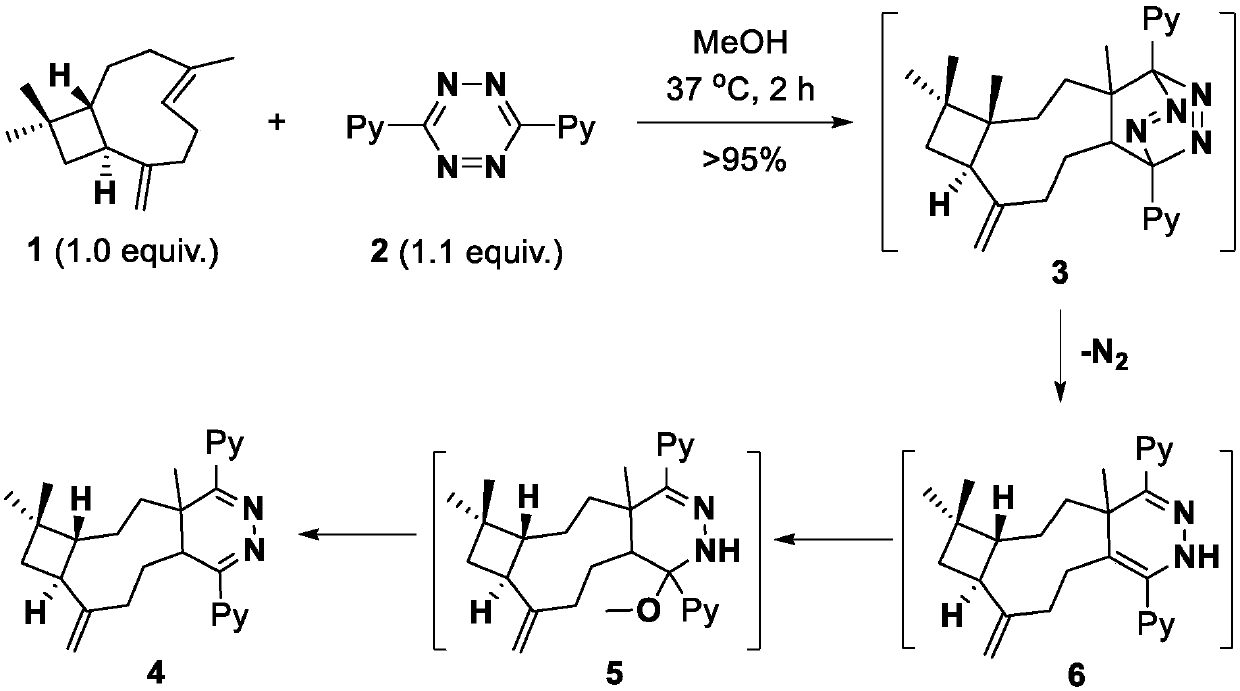
[0066] For the reaction flow chart, see figure 2 .
[0067] intermediate
[0068] (6aR,8aS)-1-methoxy-4a,7,7-trimethyl-9-methylene-1,4-di(pyridin-2-yl)-2,4a,5,6,6a,7,8,8a ,9,10,11,11a-dodecahydro-1H-cyclobuta[5,6]cyclonona[1,2-d]pyridazine(5) Synthesis
[0069] For the reaction flow chart, see figure 2 .
[0070]
[0071] Compound 1β-caryophyllene (33.7mg, 0.165mmol) was dissolved in dry methanol solution (3ml), and tetrazine compound 2 (35.3mg, 0.150mmol) was added thereto at room temperature. Stirred at room temperature for four hours, the yellow color faded, indicating The tetrazine raw material is consumed. The solvent was suspended under reduced pressure, and the residue was separated by column chromatography (dichloromethane:methanol=20:1, v / v) to obtain 53.2 mg of a light yellow oily liquid.
[0072] Structural Confirmation Data: 1 H NMR (400MHz, MeOD) δ8.61 (dd, J = 4.9, 0.9Hz, 1H), 8.55 (d, J = 4.5Hz, 1H), 7.90–7.85 (m, 2H), 7.73 (d, J = 8.0Hz,1H),7.46–7....
Embodiment 2
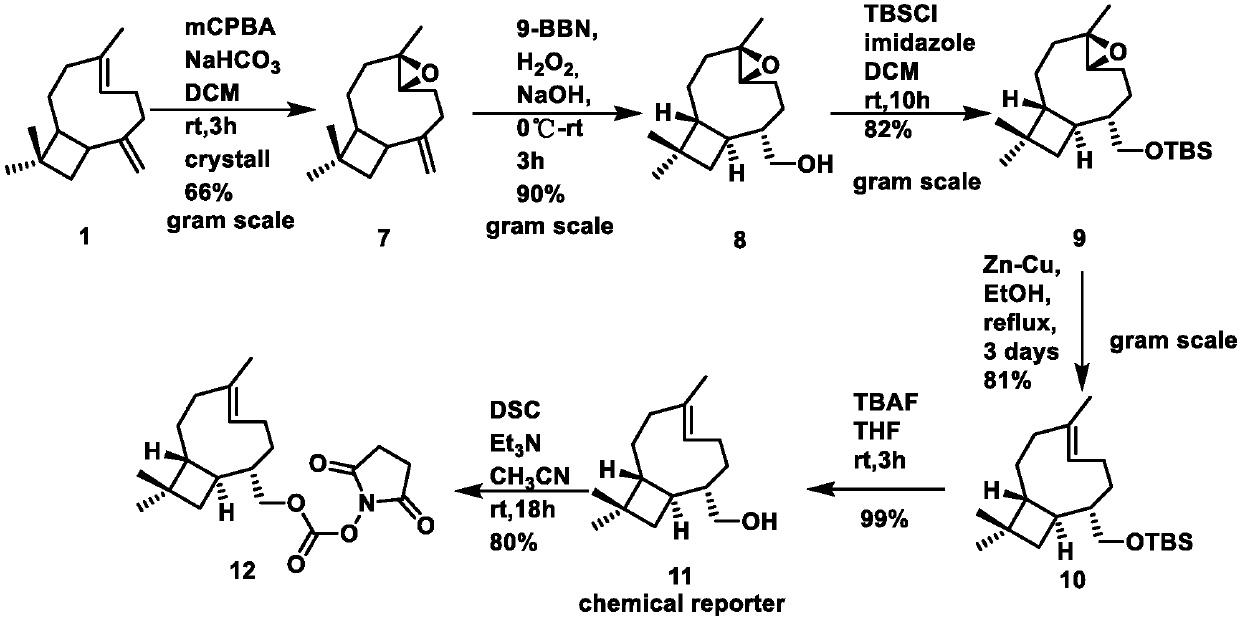
[0079] The synthesis of embodiment 2 compound 12
[0080]
[0081] Compound (4R,6R)-4,12,12-trimethyl-9-methylene-5-oxatricyclo[8.2.0.0 4,6 Synthesis of ]dodecane(7)
[0082] Its reaction flow chart sees image 3
[0083]
[0084] Compound 1β-caryophyllene (5.5ml, 5g, 24.45mol) was dissolved in dry dichloromethane solution (35ml), and 15% NaHCO was added at room temperature 3 (68ml), after stirring evenly, slowly add mCPBA (7.4g, 36.675mmol), after stirring for two hours, quench the reaction with 15% NaOH solution (100ml), the organic phase was washed with saturated brine (100ml×3), the obtained organic The phase was dried over anhydrous sodium sulfate and recrystallized to obtain 3.48 g of white solid (compound 7), with a yield of 66%.
[0085] Structural Confirmation Data: 1 H NMR (400MHz, CDCl 3 )δ4.97(s,1H),4.86(s,1H),2.88(dd,J=10.6,4.2Hz,1H),2.62(dd,J=18.8,9.5Hz,1H),2.34(ddd,J =12.6,8.1,4.4Hz,1H),2.25(ddd,J=16.7,8.1,4.3Hz,1H),2.15–2.05(m,2H),1.76(t,J=9.9Hz,1H),...
Embodiment 3
[0103] Example 3, Cytotoxicity Test of β-Caryophyllene and Its Derivatives (Compound 11)
[0104] Hela cells were incubated with different concentrations of β-caryophyllene and compound 11 (5 μM, 10 μM, 25 μM, 50 μM, 100 μM, 250 μM, 500 μM) in methanol solution, and after 24 hours, the cell survival was measured by the MTT method. The experimental results are as follows Figure 4 As shown, this experiment was repeated three times.
[0105] Depend on Figure 4 It can be seen that at the highest concentration of 500 μM, there is still no obvious adverse effect on the growth of cells. This experiment proves that β-caryophyllene and its derivatives are slightly toxic as reactants.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com