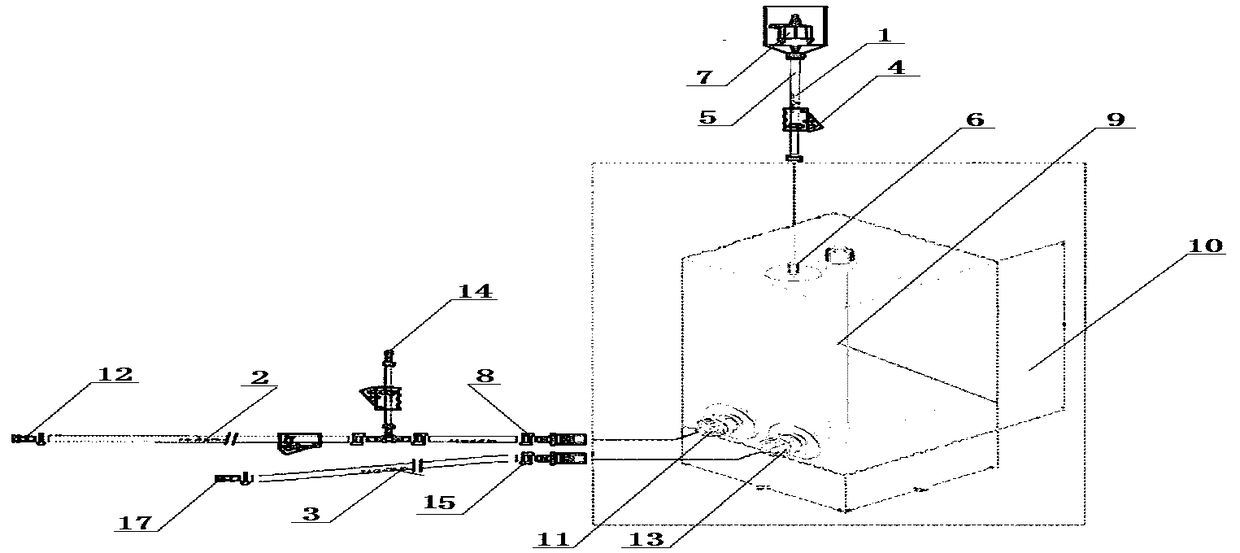
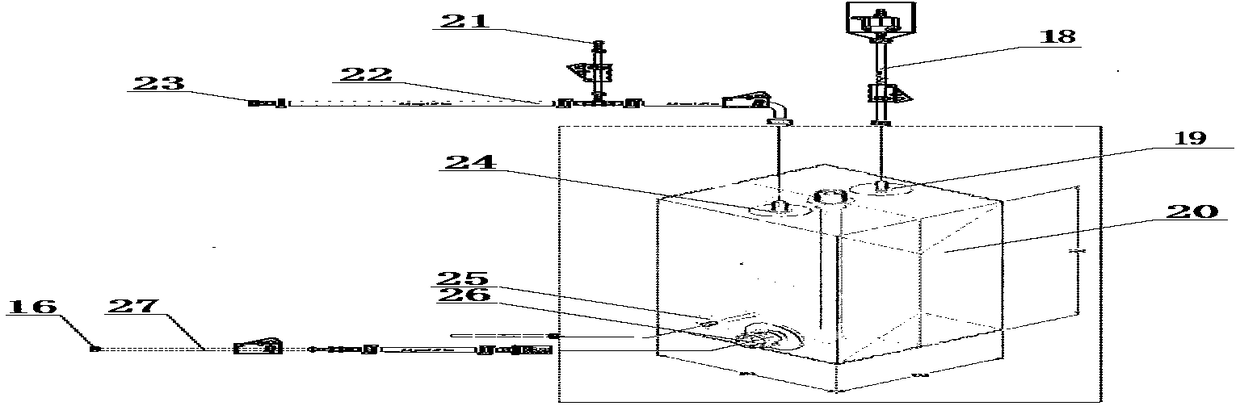
A device and method for inactivating rabies virus and hydrolyzing an inactivating agent using a disposable bag
A rabies virus and virus inactivation technology, which is applied in the direction of viruses, virus/bacteriophage, biochemical cleaning equipment, etc., can solve the problems of antigen loss and insufficient inactivation, and achieve low risk of damage, reduce material splash, reduce The effect of antigen loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The large-scale inactivation method of embodiment 1 rabies vaccine virus liquid:
[0041] Take the prepared Vero cell rabies virus harvest liquid and filter it through 0.8μm and 0.45μm microporous filters. After clarification and filtration, use Millipore's 300KD ultrafiltration membrane bag for ultrafiltration. The washing liquid (BME liquid, containing 0.02% glutamine by mass percentage, 24.63% sodium bicarbonate) is used to balance the membrane package, and the pH value of the reflux liquid is detected, and the pH value is 6.0. After meeting the requirements, it is concentrated, and the concentration ratio is 25 times. Transfer the virus pool from port A to the inactivated disposable bag 10. First dilute β-propiolactone with sterile water for injection 1:40 times, and then add it to the concentrated virus solution from port B of the bag according to the volume ratio of 1:100, and use PBS solution to wash from port B. Close the EZD valve A8, place in a cold storage a...
Embodiment 2
[0042] Embodiment 2 Inactivator hydrolysis situation:
[0043] The hydrolysis of β-propiolactone at 37°C was monitored by gas chromatography analysis method. Analysis of hydrolysis of β-propiolactone. Through the analysis of the test results, the concentration of β-propiolactone dropped from 299.4ppm when it was not hydrolyzed to 34.4ppm when it was hydrolyzed for 150min, which was lower than the quantitative limit of the method, indicating that β-propiolactone could be completely hydrolyzed at 37°C for 150min. hydrolysis effect.
Embodiment 3
[0044] Embodiment 3 cell method verification inactivation effect:
[0045] According to the inactivation method of the embodiment, take the virus stock solution according to every 3cm 2 Inoculate the inactivated rabies virus liquid into MRC-5 cells at a ratio of 1ml for cell inoculation, culture for 7 days and then subculture, continue to culture for 21 days, inoculate the cell suspension on Lab-tek chamber slides (purchased from Thermo Company), After 3-4 days, Lab-tek was observed for the presence of rabies virus by immunofluorescence. Among them, the cell maintenance solution (MEM solution containing 4% fetal bovine serum) was replaced on the 14th day and 21st day respectively, and the cell supernatant was collected for mouse brain cavity poisoning, and the mice were observed for 21 days. A cell negative control and a virus positive control were also set up in the experiment to determine the validity of the experiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com