A photoelectric compound containing thiophene (selenophene) modification and its preparation method and application
A compound and optoelectronic technology, applied in the field of material chemistry, can solve the problem of few materials and achieve the effect of high open circuit voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: Synthesis of optoelectronic compounds M1, M2, M3 and M4.
[0109] A kind of organic photoelectric compound that is used for solar cell, the chemical structure of its compound is as shown in formula (I), and wherein several more specific structures are as follows:
[0110]
[0111] where X 1 =X 2 =X 3 =X 4 =S, R 2 = R 4 =C 6 h 13 , R 3 The specific structure after =H is as follows:
[0112]
[0113]
[0114] Preparation and synthesis of optoelectronic compound M1
[0115] It mainly includes the following steps:
[0116] ①Synthesis of π-bridge monomer 5-(5-bromo-6-hexyl[3,2-b]thiophen-2-yl)-4-hexylthiophene-2-carbaldehyde:
[0117]
[0118] ②Synthesis of intermediate dialdehyde terminal group and target compound M1
[0119]
[0120] ③Concrete steps for the synthesis of each compound:
[0121] Compound 3: Under nitrogen protection, Pb(PPh 3 ) 4 (5%mmol) was added to compound 1 (3g, 8.57mmol), compound 2 (2.5g, 9.12mmol), NaHCO 3 ...
Embodiment 3
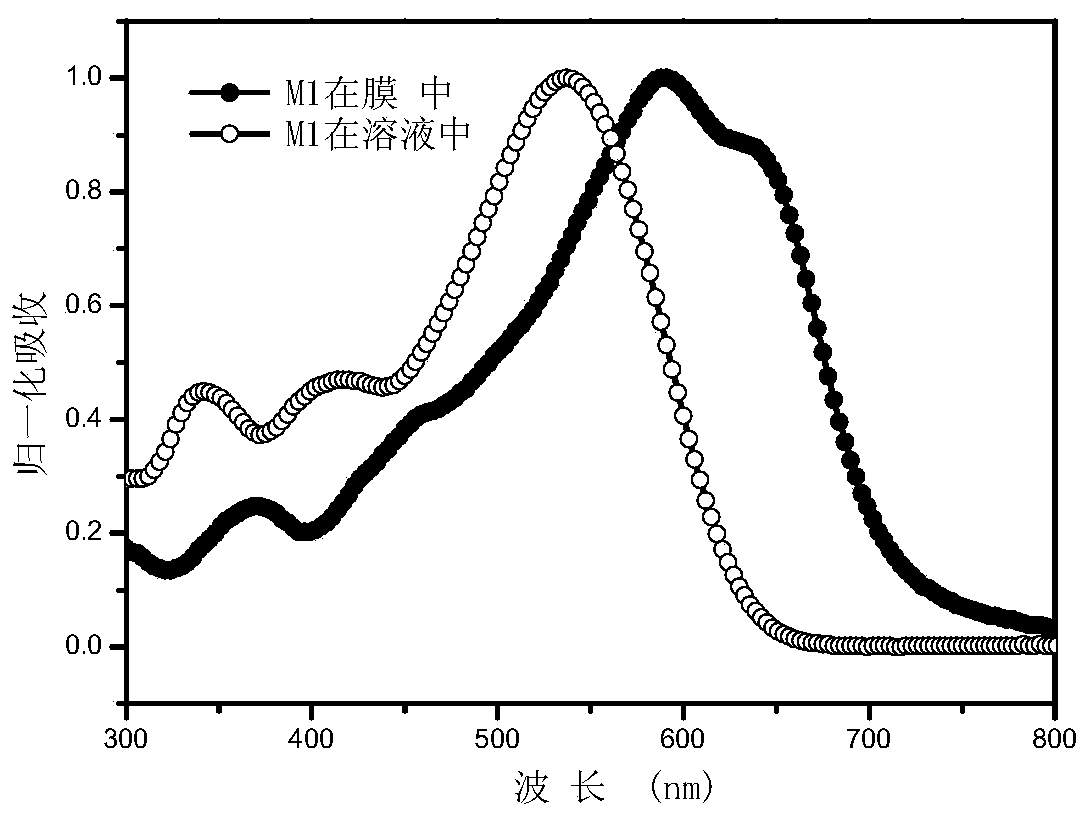
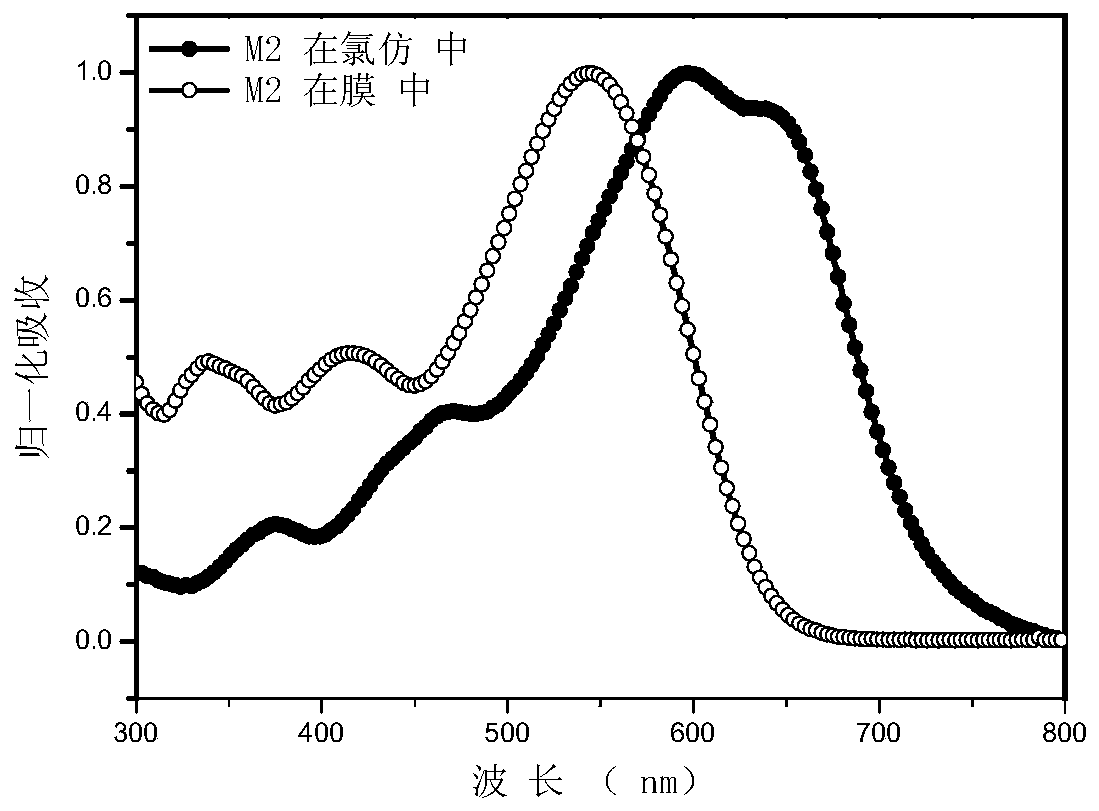
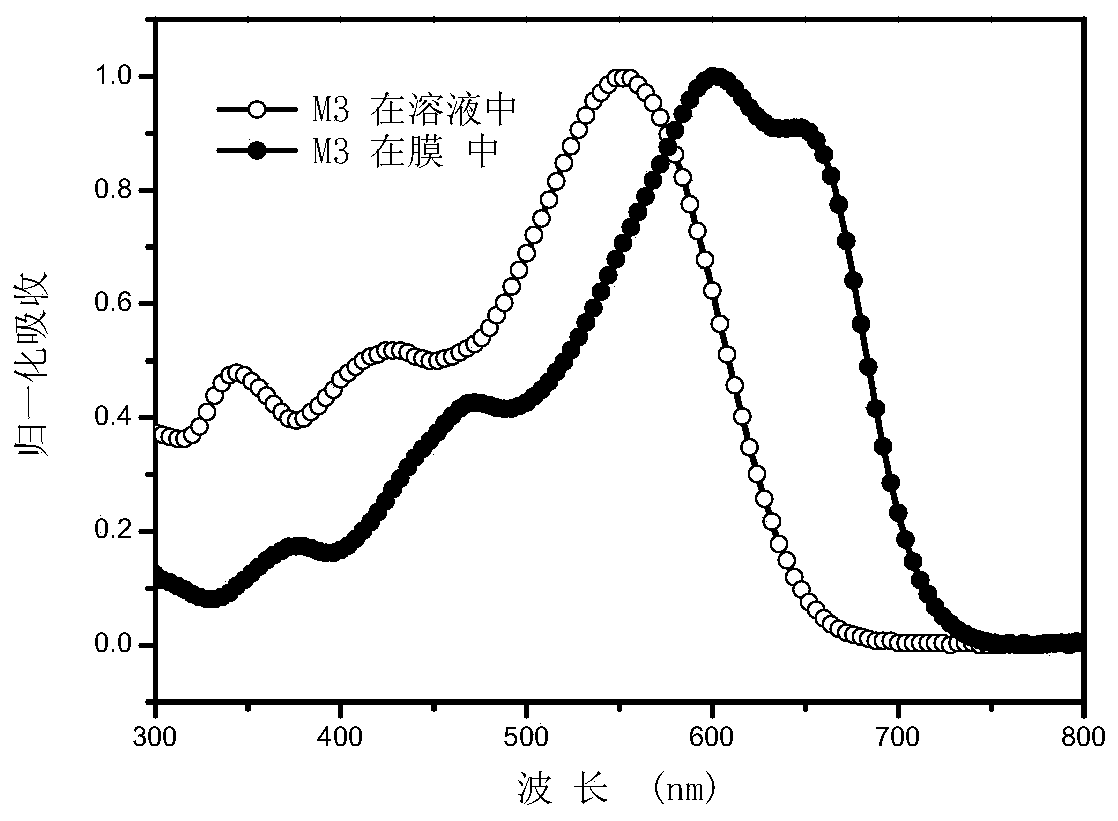
[0136] Embodiment 3: Determination of the ultraviolet-visible absorption spectra of small molecules M1-M4 in chloroform solution and film state.
[0137] Dissolve an appropriate amount of M1 or M2 in chloroform to make a solution with a certain concentration, and take part of the solution and spin-coat it on a quartz plate to form a thin film of small molecules. The ultraviolet-visible absorption spectra of the compounds of M1-M4 measured in chloroform solution and film state are as follows: Figure 1-4 shown. It can be seen from the figure that M1-M4 has a wide absorption in the visible light region, and the film has a red shift of about 80nm relative to the solution, indicating that the two have a good aggregation effect in the film.
Embodiment 4
[0138] Embodiment 4: Determination of the cyclic voltammetry curve in the state of small molecule film
[0139] Figure 5 is based on the cyclic voltammograms of M1 and M2 films, Figure 6 Cyclic voltammograms based on M3 and M4 thin films. The chloroform solution was coated on the platinum electrode, and Ag / Ag+ was used as the reference electrode, and after drying to form a film, it was placed in the acetonitrile solution of tetrabutylammonium hexafluorophosphate for measurement. The onset oxidation potential and onset reduction potential obtained from the figure are then given by the formula E HOMO =-e(Eonset ox+4.71)(eV), ELUMO=-e(Eonset red+4.71)(eV) Calculate the HOMO and LUMO energy levels of these two compounds. See Table 1 for specific values. It can be seen from the table that the four materials all have lower highest electron-occupied orbitals (HOMO), which provides a basis for obtaining high open circuits for photovoltaic devices prepared based on these two mate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com