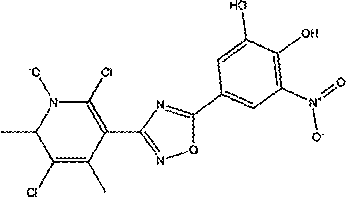
Opicapone compound
A technology of Opica and composition, applied in the field of medicine, can solve problems such as unfavorable drug quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
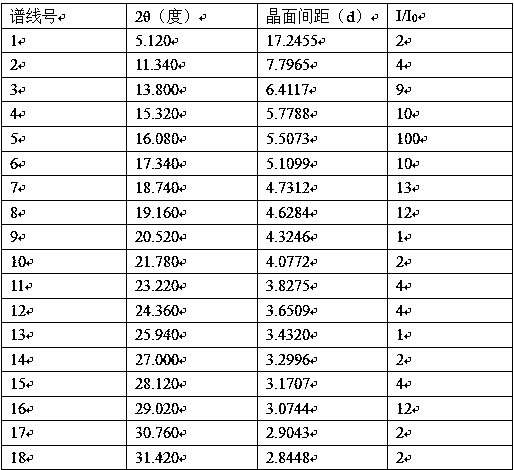
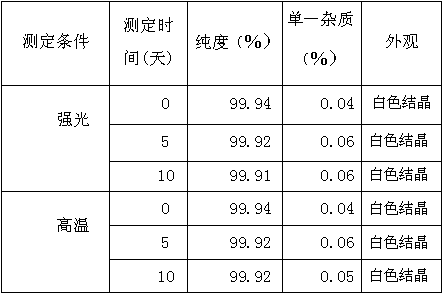
[0030] In the reaction bottle, add 10 grams of opicapone and 110ml of methane-ethanol-water (8:2:0.2) mixture, heat to 61°C-64°C, stir for 20 minutes, filter while hot, 38°C- 40°C, heat preservation for 3 hours, then lower the temperature, heat preservation at 17°C-21°C for 9 hours, filter, and dry to obtain the above-mentioned opimcapone crystals with a purity of 99.94%.
Embodiment 2
[0032] In the reaction bottle, add 10 grams of opicapone and 100ml of methane-ethanol-water (9:1:0.1) mixture, heat to 61°C-64°C, stir for 20 minutes, filter while hot, 38°C- 40°C, heat preservation for 3 hours, then lower the temperature, heat preservation at 17°C-21°C for 9 hours, filter, and dry to obtain the above-mentioned opimcapone crystals with a purity of 99.96%.
Embodiment 3
[0034] Tablets containing Opicapone
[0035] Prescription: 4.41 grams of opicapone crystals, 195 grams of lactose, 45 grams of PEG-4000, 18 grams of magnesium stearate, 48 grams of croscarmellose sodium, appropriate amount of distilled water, made into 1000 tablets.
[0036] Process: Grind PEG-4000 and Opicapone crystals together, pass through 80-mesh sieve, mix with other materials, use distilled water to make soft material, 16-mesh sieve to make granules, dry in a drying oven at 40-45°C, 16 mesh Sieve the granules, add magnesium stearate to the dry granules, mix well, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com