Method for measuring ethyl p-toluenesulfonate as genetic toxicity impurities in ibuprofen
A technology of ethyl toluenesulfonate and genotoxicity, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems such as the few methods of measuring ethyl toluenesulfonate, and achieve good specificity, short time consumption and low detection cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
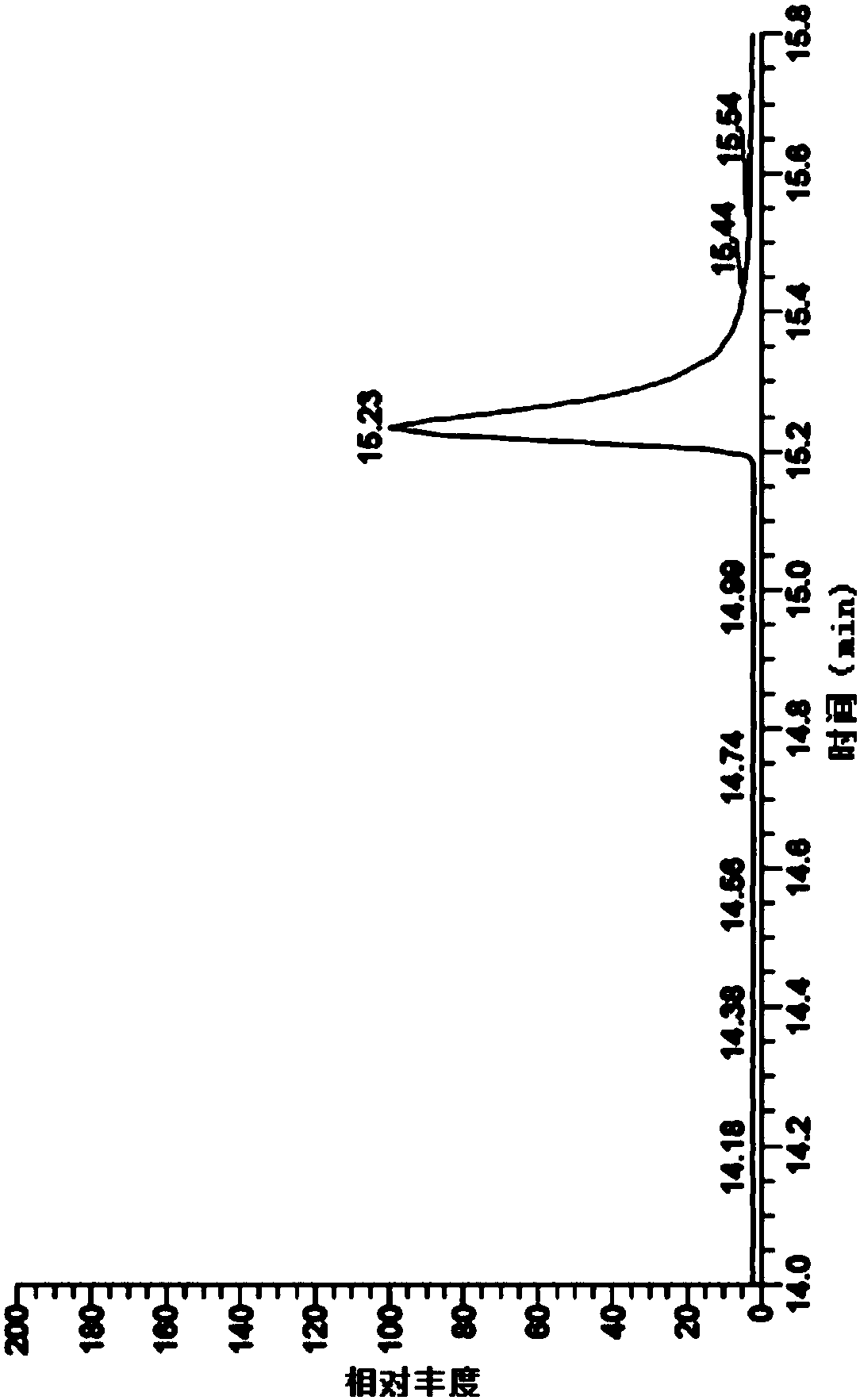
[0060] The detection of genotoxic impurity ethyl p-toluenesulfonate in embodiment 1 ibuprofen
[0061] 1) Gas chromatography and mass spectrometry conditions:
[0062] Chromatographic column: TG-5MS (30m×0.32mm, 0.25μm);
[0063] Heating program: keep at 50°C for 2 minutes, rise to 200°C at 10°C / min, then rise to 300°C at 30°C / min and hold for 10 minutes;
[0064] Carrier gas: helium, constant flow mode;
[0065] Flow rate: 1.0mL / min;
[0066] Injection port: 280°C, splitless injection, splitless time 1min;
[0067] Injection volume: 1μl;
[0068] MS: EI source, selected ion scan mode, selected ions are m / z: 91, 92, 155;
[0069] Ion source temperature: 280°C;
[0070] Transmission line temperature: 280°C;
[0071] Solvent delay time: 5min;
[0072] 2) Solution preparation
[0073] Get 5g of ibuprofen, accurately weighed, put in a 10ml measuring bottle, add acetone to dissolve and dilute to the mark, shake well, as the test solution. Take an appropriate amount of eth...
Embodiment 2
[0076] The detection of genotoxic impurity ethyl p-toluenesulfonate in embodiment 2 ibuprofen
[0077] 1) Gas chromatography and mass spectrometry conditions:
[0078] Chromatographic column: TG-5MS (30m×0.32mm, 0.25μm);
[0079] Heating program: keep at 50°C for 2 minutes, rise to 200°C at 10°C / min, then rise to 300°C at 30°C / min and hold for 10 minutes;
[0080] Carrier gas: helium, constant flow mode;
[0081] Flow rate: 1.0mL / min;
[0082] Injection port: 280°C, splitless injection, splitless time 1min;
[0083] Injection volume: 1.0μl
[0084] MS: EI source, selected ion scan mode, selected ions are m / z: 91, 92, 155;
[0085] Ion source temperature: 280°C;
[0086] Transmission line temperature: 280°C;
[0087] Solvent delay time: 5min;
[0088] 2) Solution preparation
[0089] Get 2g of ibuprofen, accurately weighed, put in a 10ml measuring bottle, add n-hexane to dissolve and dilute to the mark, shake well, and use it as the test solution. Take an appropriate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Caliber | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com