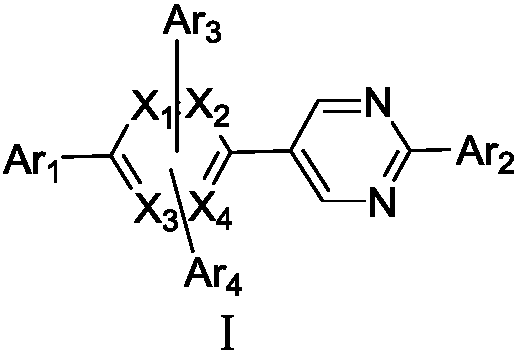
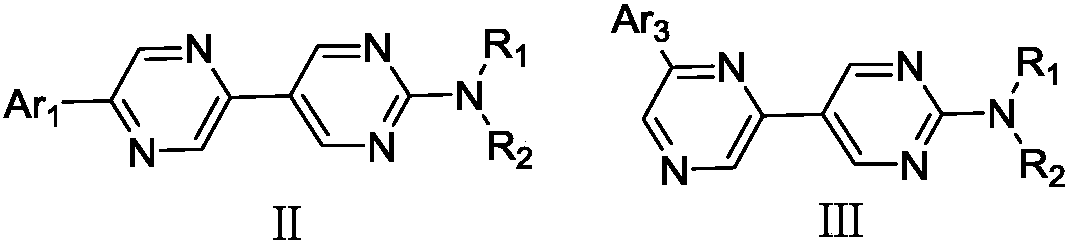
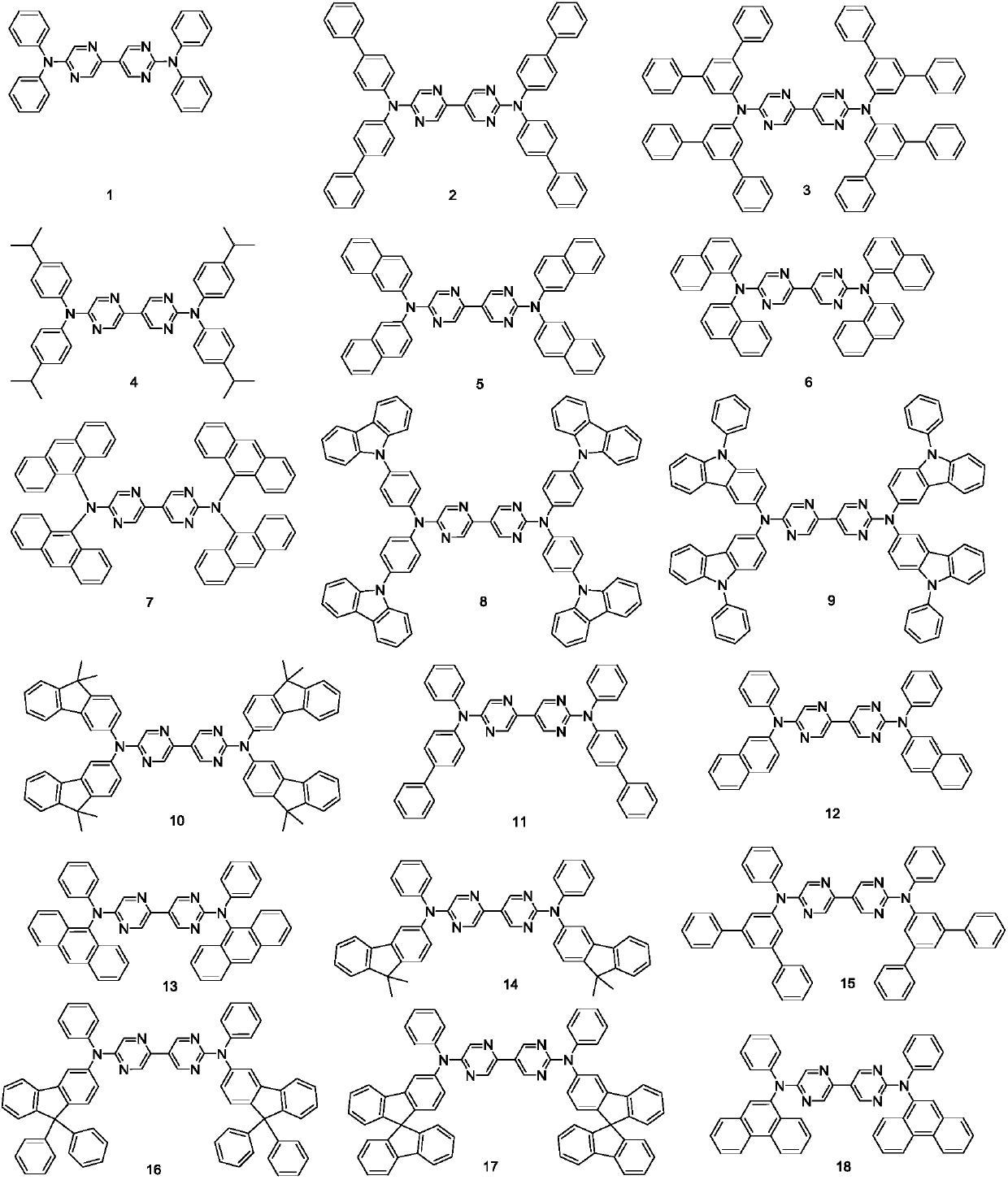
Pyrazine united pyrimidine derivative and its organic lighting device
An organic light-emitting device, pyrazine bipyrimidine technology, applied in the field of pyrazine bipyrimidine derivatives and organic light-emitting devices, can solve the problems of unbalanced recombination of electrons and holes in the light-emitting layer, fast device loss, and low luminous efficiency. Achieve good bipolar transmission performance, good luminous efficiency and lifetime performance, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] [Example 1] Synthesis of Compound 1
[0058]
[0059] Step1: Add 5-(5-bromopyrazin-2-yl)pyrimidin-2-amine (2.18g, 8.4mmol), bromobenzene (2.86g, 18.2mmol), potassium tert-butoxide (2.83g, 25.2mmol), Pd 2 (dba) 3 (0.07g, 0.08mmol), 40mL of ultrasonically deoxygenated xylene, stirred and dissolved, replaced the air three times, added P(t-Bu) 3 (0.07g, 0.34mmol), replace the air three times again, reflux for 6h, cool to room temperature, add enough dichloromethane to make the product dissolve completely, pass through a small amount of silica gel funnel, remove catalyst and salt, and concentrate the filtrate to viscous , through column chromatography to obtain intermediate 1-1 (2.24g, 66%).
[0060] Step2: Add intermediate 1-1 (3.40g, 8.4mmol), diphenylamine (1.56g, 9.2mmol), potassium tert-butoxide (2.83g, 25.2mmol), Pd into the reaction vessel 2 (dba) 3 (0.07g, 0.08mmol), 40mL of ultrasonically deoxygenated xylene, stirred and dissolved, replaced the air three tim...
Embodiment 2
[0061] [Example 2] Synthesis of compound 2
[0062] According to the synthetic method of compound 1, compound 2 (4.28 g, 64%) was obtained.
[0063]
Embodiment 3
[0064] [Example 3] Synthesis of compound 19
[0065]
[0066] Step1: add aniline (0.75g, 8mmol), 2-bromo-triphenylene (2.05g, 6.67mmol), Pd in the reactor 2 (dba) 3 (0.17g, 0.2mmol), P(t-Bu) 3 (0.14g, 0.67mmol), NaOt-Bu (2.24g, 20mmol), 100mL of toluene solution, reacted at 100°C for 24h, extracted the organic phase with diethyl ether and water after the reaction, and washed the organic layer with MgSO 4 Drying, concentration of the organic matter, column chromatography and recrystallization gave intermediate 19-1 (1.58 g, 74%).
[0067] Step2: Add 5-(5-bromopyrazin-2-yl)pyrimidin-2-amine (2.18g, 8.4mmol), bromobenzene (1.32g, 8.4mmol), potassium tert-butoxide (2.83g, 25.2mmol), Pd 2 (dba) 3 (0.07g, 0.08mmol), 40mL of ultrasonically deoxygenated xylene, stirred and dissolved, replaced the air three times, added P(t-Bu) 3 (0.07g, 0.34mmol), replace the air three times again, reflux for 6h, cool to room temperature, add enough dichloromethane to make the product disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com