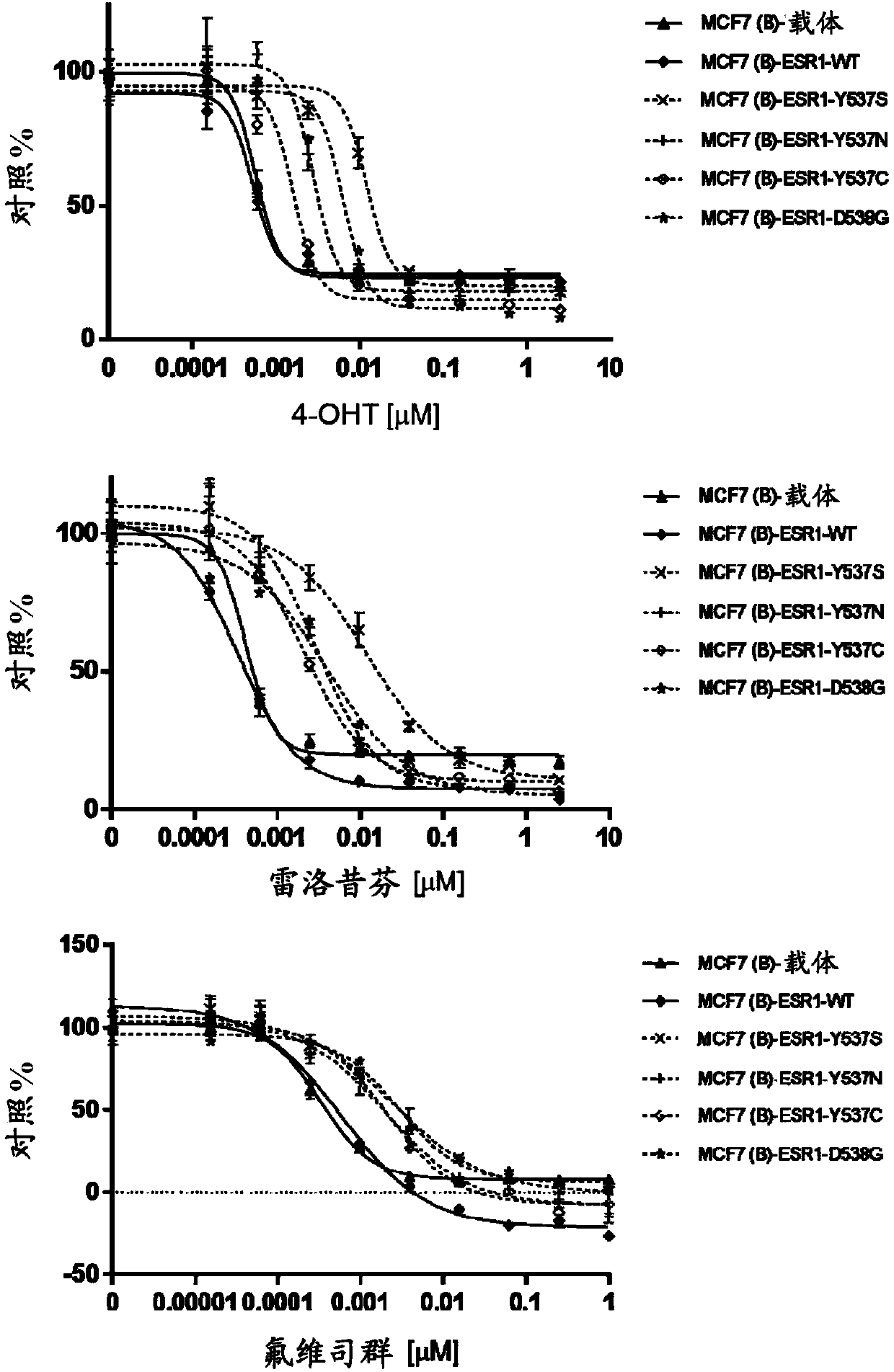
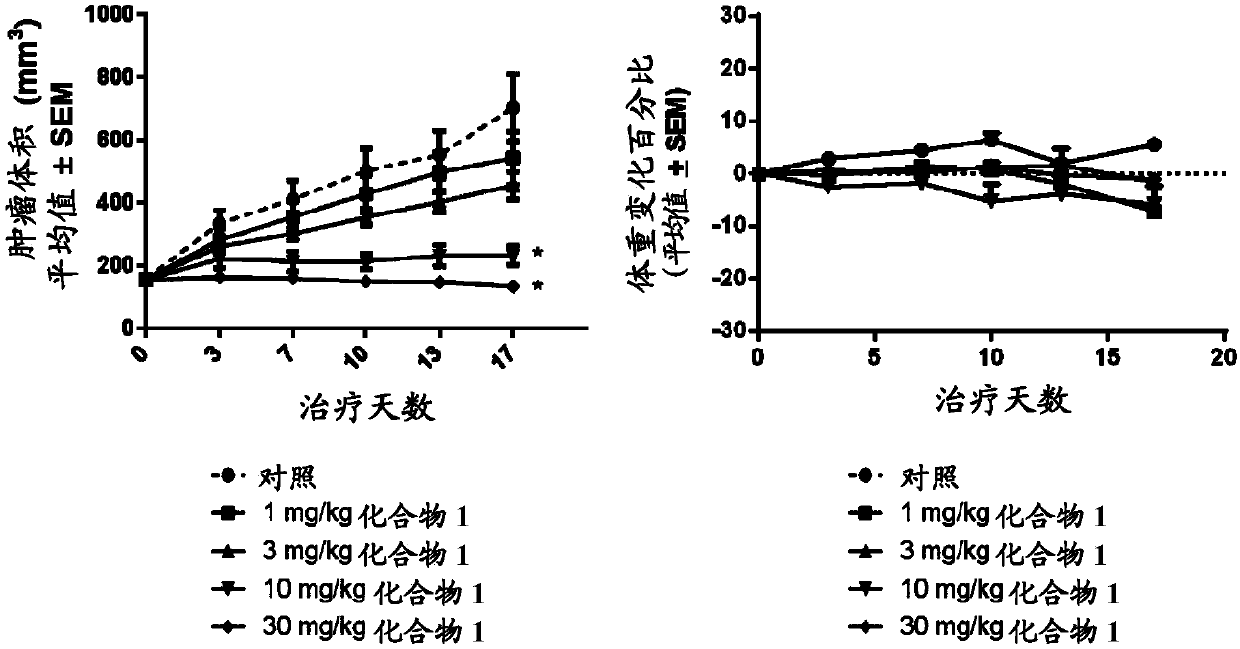
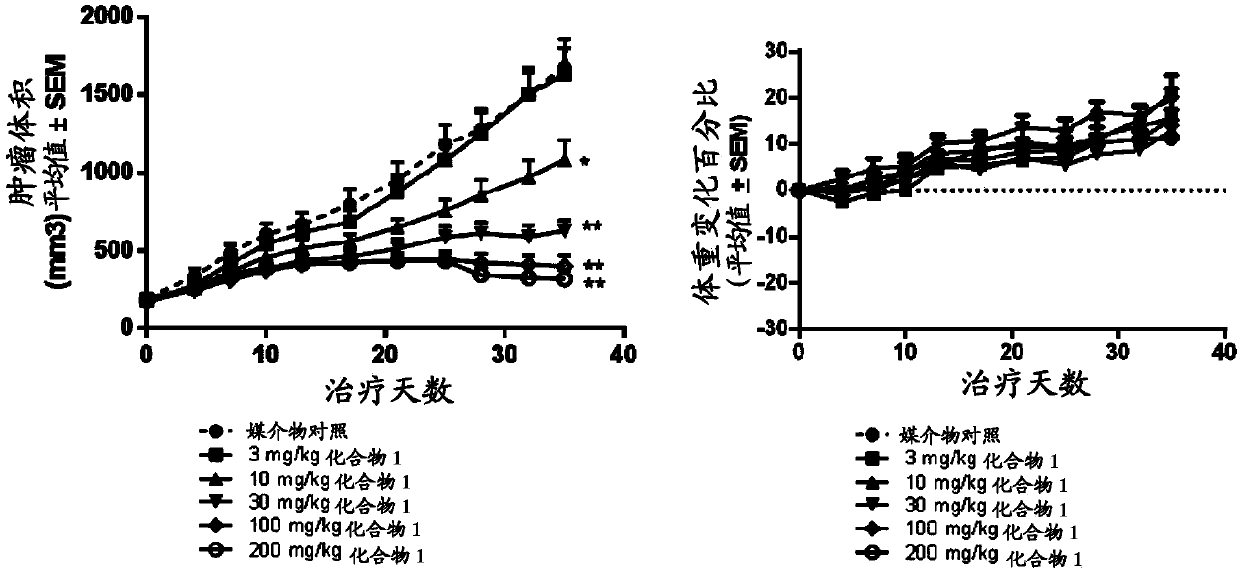
Tetrasubstituted alkene compounds and their use
A compound, enamide technology, applied in the field of tetra-substituted olefin compounds and their uses, which can solve problems such as loss of bone density, increased risk of endometrial cancer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 101
[0147]
[0148]
[0149]
[0150]
[0151]
[0152]
[0153]
[0154]
[0155]
[0156]
[0157]
[0158]
[0159]
[0160] General procedure
[0161] The following abbreviations can be used here:
[0162] ACN: Acetonitrile
[0163] BOC: tert-butoxycarbonyl
[0164] CAN: Ammonium cerium nitrate
[0165] Conc.: concentrated
[0166] Cs 2 CO 3 : cesium carbonate
[0167] DABCO: 1,4-diazabicyclo[2.2.2]octane
[0168] DCM: dichloromethane
[0169] DHP: dihydropyran
[0170] DIPEA: N,N-diisopropylethylamine, Hunig's base
[0171] DMA: Dimethylacetamide
[0172] DMF: Dimethylformamide
[0173] DMSO: Dimethylsulfoxide
[0174] DPEphos: (oxydi-2,1-phenylene)bis(diphenylphosphine)
[0175] EDCI.HCl: N-(3-Dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride
[0176] EtOH: ethanol
[0177] EtOAc: ethyl acetate
[0178] Et 3 N: Triethylamine
[0179] Ex.: Example
[0180] h: hours
Embodiment 1
[0248] Example 1: (E)-4-((2-(4-((E)-1-(1H-indazol-5-yl)-2-phenylbut-1-en-1-yl)benzene Synthesis of Oxy)ethyl)amino)-N,N-Dimethylbut-2-enamide (Compound 1)
[0249] Step 1: Synthesis of 5-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
[0250]
[0251] According to Scheme 1 step 1, the reaction was carried out with 5-bromo-1H-indazole (5 g, 23.5 mmol) instead of compound 201. Purification of the crude material by column chromatography on 230-400 mesh silica using 4-5% ethyl acetate in hexane afforded the title compound of Example 1, step 1 as a pale yellow oil (12.6 g, 86 %).
[0252] Step 2: Synthesis of but-1-yn-1-yltrimethylsilane:
[0253]
[0254] To a stirred solution of (trimethylsilyl)acetylene (116 g, 1.19 mol) in anhydrous THF (400 mL) was added n-BuLi (2.5 M in THF) at -78 °C over 2 hours. , 500mL). The resulting mixture was warmed to 0 °C for 10 min. The reaction mixture was cooled to -78°C again, HMPA (234 g, 1.13 mol) was added to the above mixture, an...
Embodiment 1A
[0282] Example 1A - Synthesis of the Hydrochloride Salt of Compound 1
[0283] Step 1A: Synthesis of 5-bromo-1-(tetrahydro-2H-pyran-2-yl)-1H-indazole
[0284]
[0285] 5-Bromoindazole (11.0Kg, 0.055mol), acetonitrile (110L) and PTSA (1.0Kg, 0.055mol) were added to a clean, dry nitrogen-flushed 200L glass reactor, and the reaction mixture was heated at 28- Stir at 30°C for 30 minutes. To the above solution was added dihydropyran (7.03 Kg, 2.55 mol) dropwise over 20 minutes. The resulting mixture was stirred at room temperature for 16 hours. After completion as monitored by TLC, the reaction mixture was quenched with water (500 mL), and the acetonitrile was concentrated under reduced pressure. The obtained product was extracted with ethyl acetate (25 l x 3), the combined organic extracts were washed with water, then brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to give the desired compound, which was not After further purification, it...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com