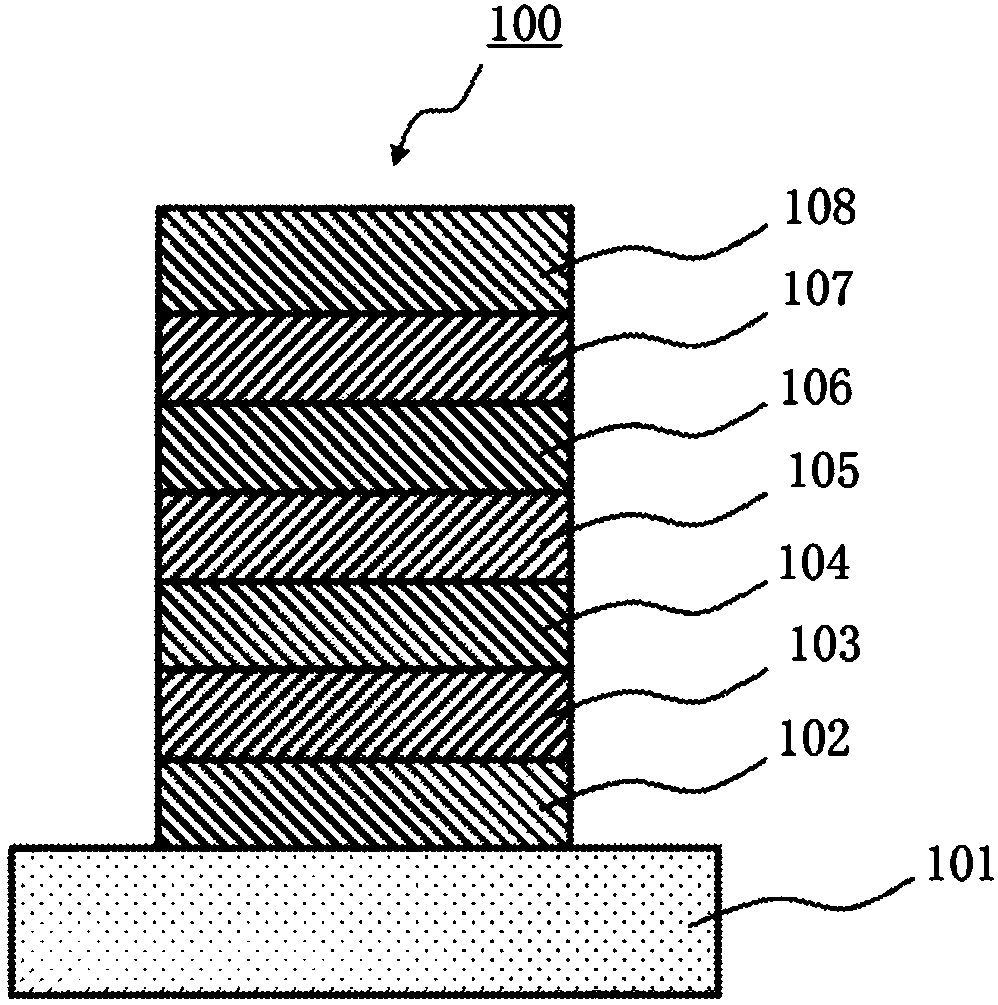
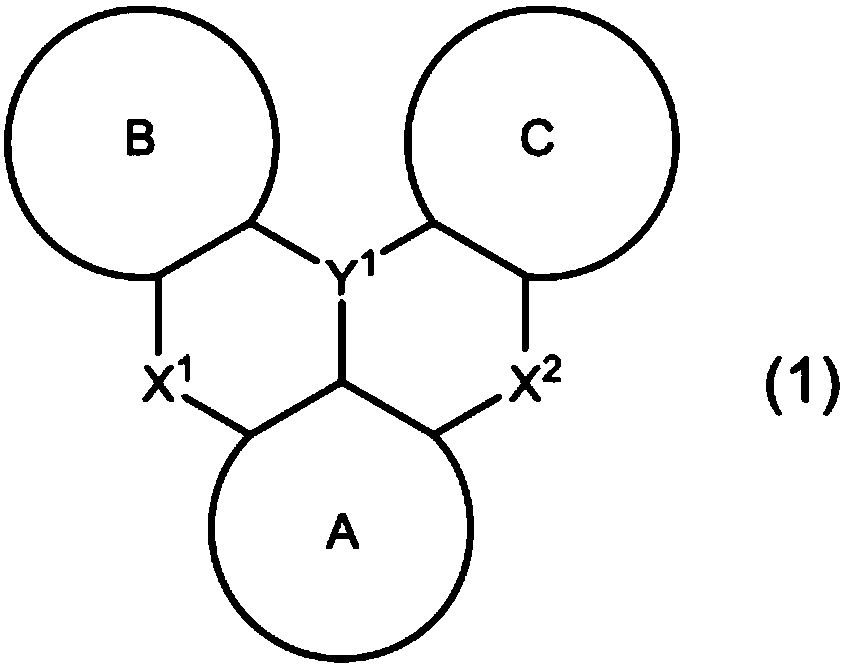
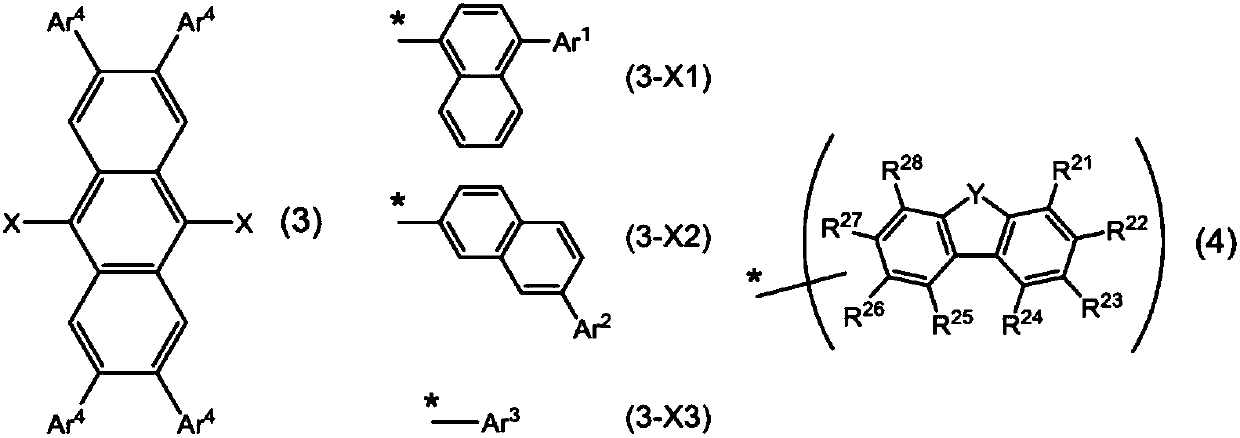
Organic electroluminescent element
一种发光元件、有机电场的技术,应用在电气元件、发光元件的半导体器件、发光材料等方向,能够解决未记载NO连结系化合物制造方法、特性未知、电子状态不同等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0559] Hereinafter, the present invention will be explained more specifically with examples, but the present invention is not limited to these examples. First, the following describes synthesis examples of polycyclic aromatic compounds and their multimers.
Synthetic example (1
[0561] Compound (1-1152): 9-([1,1'-biphenyl]-4-yl)-5,12-diphenyl-5,9-dihydro-5,9-diaza-13b- Synthesis of Bora[3,2,1-de]anthracene
[0562] [化96]
[0563]
[0564] Under a nitrogen environment, and at 80°C, diphenylamine (37.5g), 1-bromo-2,3-dichlorobenzene (50.0g), Pd-132 (Johnson Matthey) will be added ) (0.8g), NaOtBu (32.0g), and xylene (500ml) flasks were heated and stirred for 4 hours, then heated to 120°C, and further heated and stirred for 3 hours. After the reaction liquid was cooled to room temperature, water and ethyl acetate were added for liquid separation. Then, it was purified by silica gel column chromatography (developing solution: toluene / heptane = 1 / 20 (volume ratio)) to obtain 2,3-dichloro-N,N-diphenylaniline (63.0 g) .
[0565] [化97]
[0566]
[0567] In a nitrogen environment, 2,3-dichloro-N,N-diphenylaniline (16.2g), bis([1,1'-biphenyl]-4-yl)amine will be added at 120℃ (15.0g), Pd-132 (Johnson Matthey) (0.3g), NaOtBu (6.7g) and xylene (150ml) flasks were hea...
Synthetic example (2
[0576] Compound (1-422): 5,9,11,15-tetraphenyl-5,9,11,15-tetrahydro-5,9,11,15-tetraaza-19b,20b-diborin Synthesis of pentacene and [3,2,1-de:1',2',3'-jk]
[0577] [化100]
[0578]
[0579] In a nitrogen environment, 2,3-dichloro-N,N-diphenylaniline (36.0g), N 1 ,N 3 -A flask of diphenylbenzene-1,3-diamine (12.0g), Pd-132 (Johnson Matthey) (0.3g), NaOtBu (11.0g) and xylene (150ml) was heated and stirred for 3 hours. After cooling the reaction liquid to room temperature, water and ethyl acetate were added for liquid separation. Then, purification was performed by silica gel column chromatography (developing solution: toluene / heptane mixed solvent). At this time, slowly increase the ratio of toluene in the developing solution to elute the target. Furthermore, it was purified by activated carbon column chromatography (developing solution: toluene) to obtain N 1 ,N 1' -(1,3-phenylene)bis(2-chloro-N 1 ,N 3 ,N 3 -Triphenylbenzene-1,3-diamine) (22.0 g).
[0580] [化101]
[0581]
[0582] In ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron work function | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com