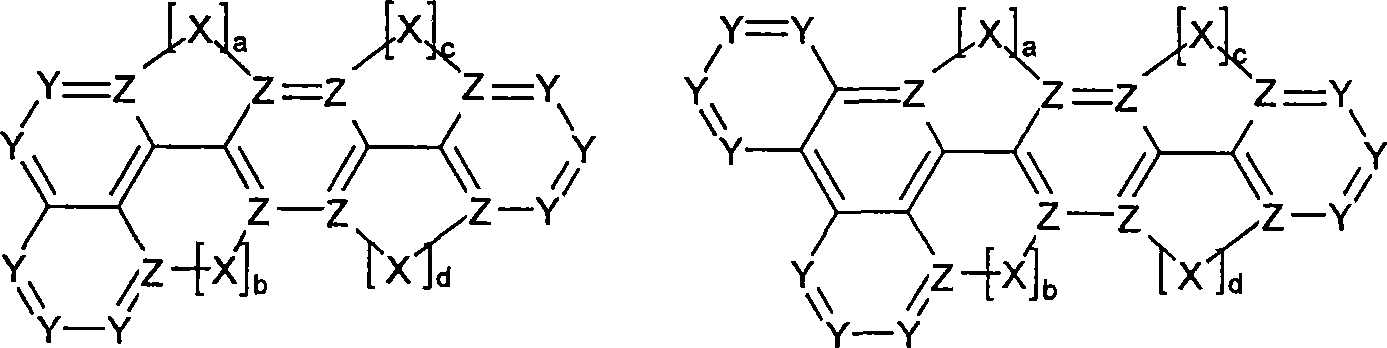
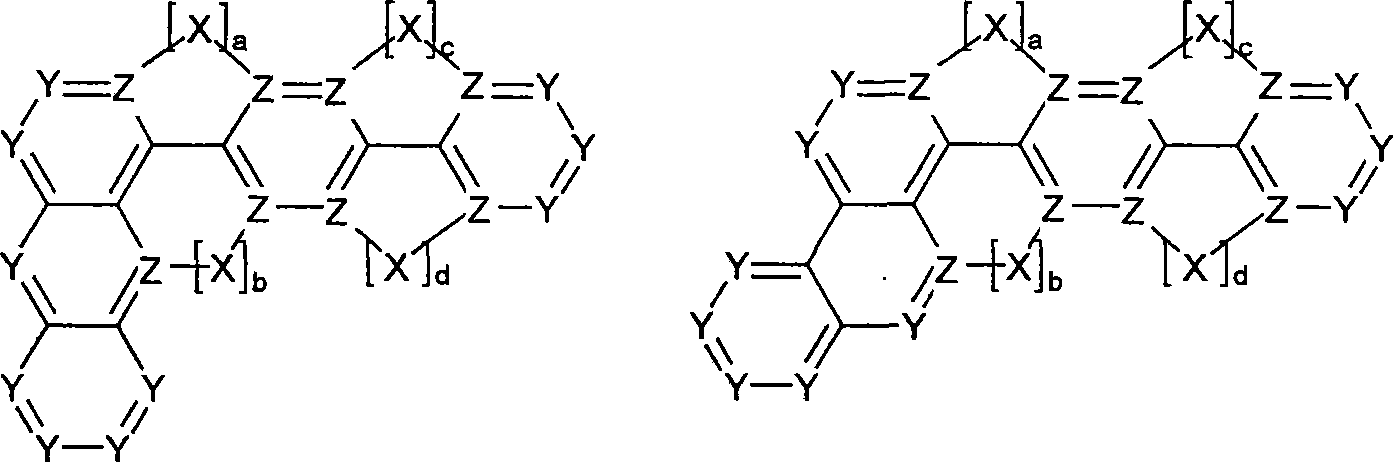
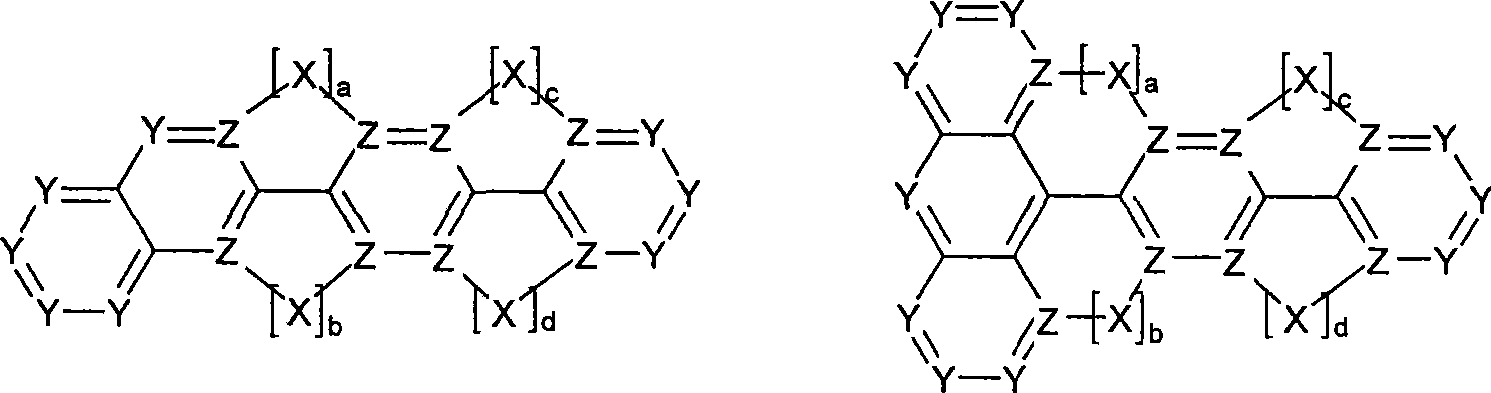
Novel materials for organic electroluminescent devices
A general formula, compound technology, applied in the field of new materials for organic electroluminescent devices, can solve the problems of low power efficiency, voltage increase, high voltage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0199] Example 1: 1,2-benzo-3,8-bis(N,N-diphenylamino)-6,6,12,12-tetramethyl-6,12-dihydroindeno[1, 2-b]fluorene
[0200] a) ethyl 1-(9,9-dimethyl-9H-fluoren-2-yl)naphthalene-2-carboxylate
[0201]
[0202] 86.3g (362.55mmol) of 9,9'-dimethylfluorene-2-boronic acid, 92g (329.59mol) of 2-carboxyethylbromonaphthalene and 159.4g (692mmol) of tripotassium phosphate monohydrate were suspended in To 450 ml of toluene, 230 ml of dioxane, 700 ml of water and 6.0 g (19.8 mmol) of tri-o-tolylphosphine, 740 mg (3.3 mmol) of palladium acetate were subsequently added, and the mixture was heated at boiling point for 4 hours. The organic phase was separated off, filtered through silica gel and evaporated in vacuo. The residue was recrystallized from heptane. Yield: 100.6 g (78%) of colorless solid.
[0203] b) 2-[1-(9,9-dimethyl-9H-fluoren-2-yl)naphthalene-2-yl]propan-2-ol
[0204]
[0205] 100.6 g of ethyl 1-(9,9-dimethyl-9H-fluoren-2-yl)naphthalene-2-carboxylate (256 mmol) was fi...
Embodiment 8
[0218] Example 8: 1,2-Benzo-3-[9-{10-(2-naphthyl)}anthracenyl]-6,6,12,12-tetramethyl-6,12-dihydroindeno [1,2-b]fluorene
[0219] a) 1,2-benzo-3-bromo-6,6,12,12-tetramethyl-6,12-dihydroindeno[1,2-b]fluorene
[0220]
[0221] Dissolve 15.5g (43mmol) of 1,2-benzo-6,6,12,12-tetramethyl-6,12-dihydroindeno[1,2-b]fluorene in 350ml of THF, add 8.4 g (47.3 mmol) of NBS, the mixture was heated at the boiling point for 4 hours. After removing the solvent in vacuo, the residue was washed by boiling in ethanol / water (1:1), the solid was filtered off with suction, washed with ethanol and dried, leaving 15.4 g (82%) of the monobromide as a colorless powder.
[0222] b) 1,2-benzo-6,6,12,12-tetramethyl-6,12-dihydroindeno[1,2-b]fluorene-3-boronic acid
[0223]
[0224] Suspend 14g (32mmol) of 1,2-benzo-3-bromo-6,6,12,12-tetramethyl-6,12-dihydroindeno[1,2-b]fluorene in 150ml of In dry ether, add 21ml (42mmol) of 2M n-butyllithium cyclohexane solution at -70°C, stir the mixture at this ...
Embodiment 13
[0231] Example 13: 1,2-benzo-3-(N,N-bis-4-tert-butylphenylamino)-6,6,12,12-tetramethyl-6,12-dihydroindeno [1,2-b]fluorene
[0232]
[0233] 23.1g (53mmol) of 1,2-benzo-3-bromo-6,6,12,12-tetramethyl-6,12-dihydroindeno[1,2-b]fluorene and 19.2g (68mmol) of di-tert-butylphenylamine was dissolved in 100ml of dry toluene, with N 2 The solution was saturated and then 0.5 ml (2 mmol) of tri-tert-butylphosphine were added, followed by 240 mg (1 mmol) of palladium acetate and 8.6 g (89 mmol) of sodium tert-butoxide. The mixture is heated at the boiling point for 3 hours, the organic phase is separated off, washed twice with water, filtered and evaporated in a rotary evaporator. Four times recrystallization from isopropanol afforded 24 g (71%) of the amine in the form of a yellow powder with a purity (RP-HPLC) of >99.9%. This compound has excellent thermal stability. No decomposition was observed upon sublimation.
[0234] The following compounds were synthesized similarly to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com