Clarifying process and quality control method of medicinal composition with function of treating dark eye circles
A quality control method, a technology for dark circles, applied in the direction of drug combination, medical preparation containing active ingredients, pharmaceutical formula, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
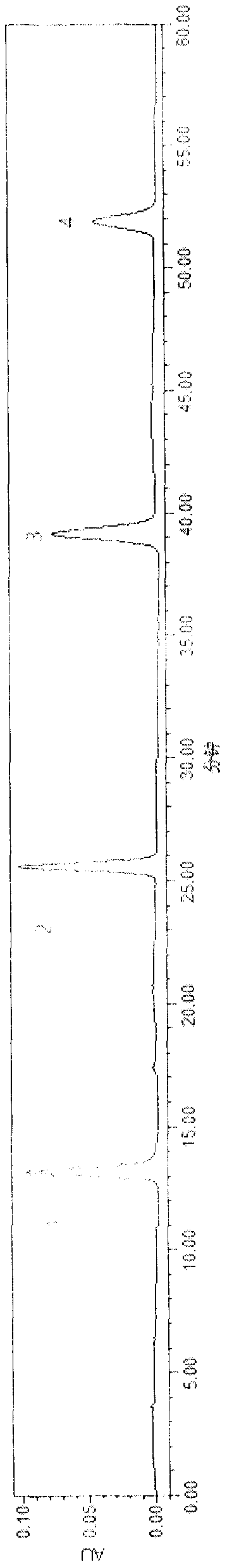
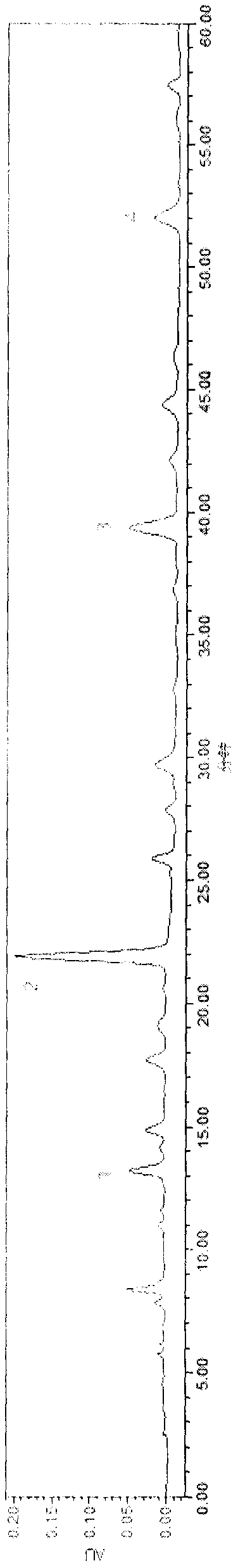
[0056] A clarification process and quality control method of a pharmaceutical composition with the effect of treating dark circles of the present invention, the clarification process is carried out as follows:
[0057] Clarification process and content of each component
[0058] (1) Preparation of clarifying agent: Type II ZTCl+1 natural clarifying agent consists of two components, A and B. Component A: Precisely measure 1.0g of component A, add a small amount of distilled water, stir to form a paste, then add distilled water to prepare 100mL of viscose, swell for 24 hours, and filter with double gauze. Component B: Precisely measure 1.0g of component B, add a small amount of 1% acetic acid solution by volume, stir to form a paste, then add 1% acetic acid solution to make 100mL viscose solution, swell for 24 hours, Filter with double gauze, that is, too.
[0059] (2) Adding order of clarifiers A and B: According to the instructions, ZTC natural clarifier mainly chooses diffe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com