Preparation method and application of enhanced Slit2 CAR-T and CAR-NK cells
A technology of CD5 and extracellular domain, applied in chemical instruments and methods, biochemical equipment and methods, animal cells, etc., can solve the problem of low specificity of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1: Preparation of lentiviral expression vector
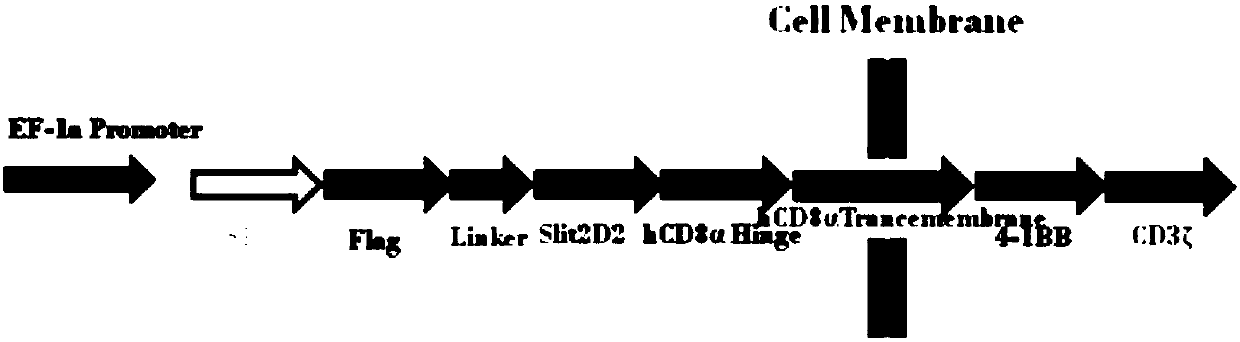
[0098] 1. According to the known Slit2 sequence [GenBank:EAW92793.1], the second structural domain Slit2D2 (Hohenester2008) of Slit2 was analyzed and designed. Hinge region of human CD8 and CD8 TM Transmembrane region gene sequence, human 4-1BB intracellular region gene sequence, CD3ζ intracellular region, IRES internal ribosome entry site, get Slit2D2-CAR (SP1-Flag-Linker-Slit2D2-CD8-4-1BB-CD3ζ) The gene is shown in SEQ ID NO: 7, and its series diagram is shown in figure 1 Shown; synthetic HAC gene fragment, its nucleotide sequence is shown in SEQ ID NO: 2, introduces HSA gene at its C terminal simultaneously, obtains HAC-HSA fusion gene (SP2-HAC-HSA) as shown in SEQ ID NO: 8 Show.
[0099] 2. Insert the above HAC-HSA fusion gene sequence into the downstream of the Slit2D2-CAR sequence to form a complete
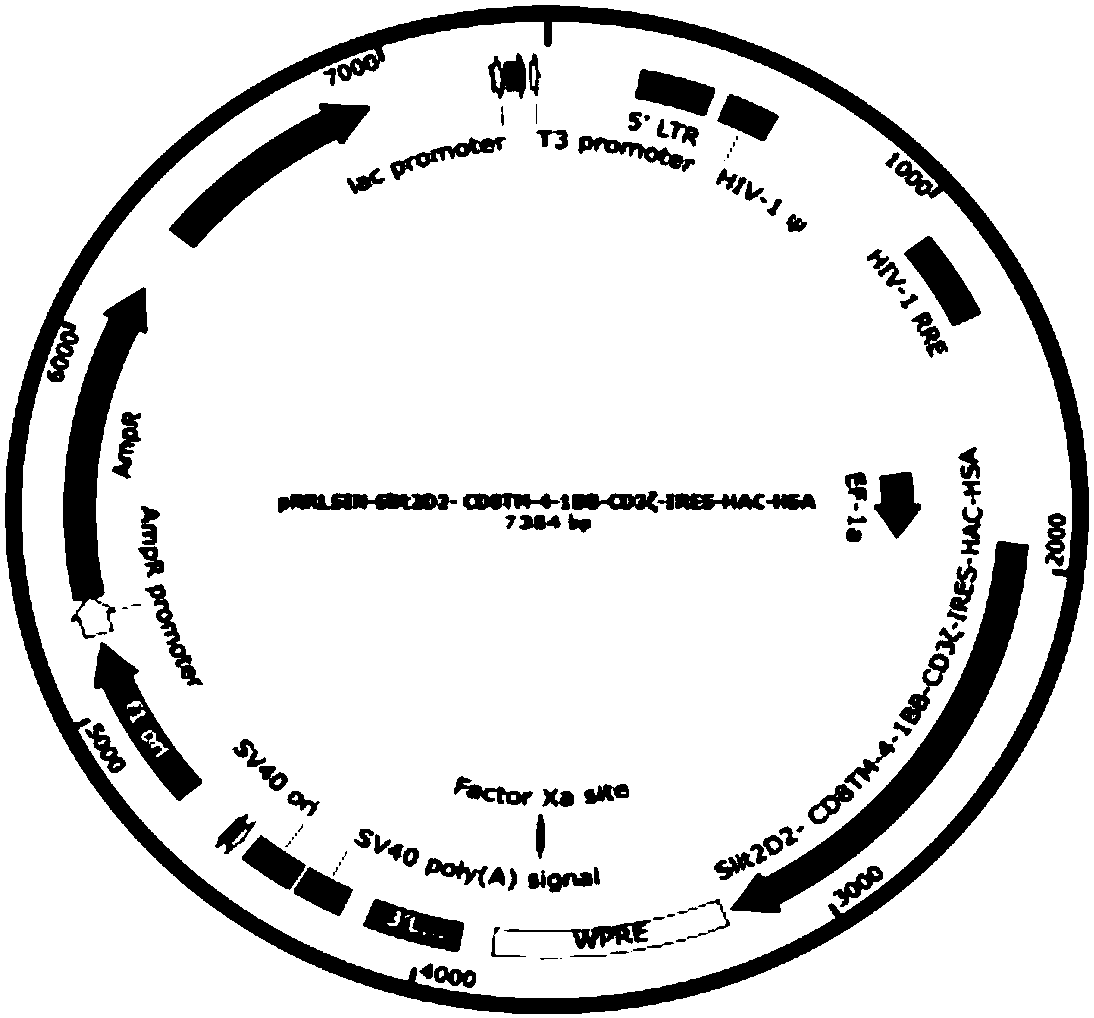
[0100] Slit2D2-CD8-4-1BB-CD3ζ-IRES-HAC-HSA, its construction schematic diagram is as follows figure 2 ...
Embodiment 2
[0102] Embodiment 2: lentivirus preparation
[0103] 1. 24 hours before transfection, use about 8×10 per dish 6 293T cells were seeded into 15cm culture dishes. Make sure that the cells are at about 80% confluence and evenly distributed in the culture dish during transfection.
[0104] 2. Prepare Solution A and Solution B
[0105] Solution A: 6.25mL 2×HEPES buffer (the amount packed in 5 large dishes is the best).
[0106] Solution B: Add the mixture of the following plasmids: 112.5 μg pRRSLIN-Slit2D2-CAR-IRES-HAC-HSA (target plasma); 39.5 μg pMD2.G (VSV-G envelope); 73 μg pCMVR8.74 (gag, pol, tat , rev); 625 μL 2M calcium ion solution. Total volume of solution B: 6.25 mL.
[0107] 3. Mix solution B well, and while vortexing solution A gently, add solution A drop by drop and let it stand for 5-15 minutes. Gently vortex the above mixed solution of A and B, add dropwise to the culture dish containing 293T cells, gently shake the culture dish back and forth to make the mixt...
Embodiment 3
[0110] Example 3: Preparation of CAR-T cells
[0111] 1. Take 0.5mL of blood for rapid detection of pathogenic microorganisms to exclude microbial infections such as HBV, HCV, HDV, HEV, HIV-1 / 2, Treponema pallidum and parasites; coagulation), immediately (4°C, within 24 hours) to the cell preparation laboratory to ensure that there is no pathogenic microorganism contamination in this process. After obtaining the patient's blood, in the GMP preparation room, wipe the surface of the heparin bottle with an alcohol cotton ball for disinfection and put it into a biological safety cabinet.
[0112] 2. Open two 50mL centrifuge tubes in advance, transfer the blood into two 50mL centrifuge tubes, and tighten; put the two 50mL centrifuge tubes filled with blood into the centrifuge, centrifuge at 400g (2000rpm) for 10min, and centrifuge at room temperature Afterwards, the upper layer of plasma was collected, leaving the precipitate layer; the collected autologous plasma was inactivated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com