Method for selective oxidation of o-chlorophenol in alkaline wastewater
An o-chlorophenol, selective technology, applied in the field of selective oxidation of o-chlorophenol, can solve problems such as inability to remove well, limited reaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1) Weigh 6.04875g of Na 2 MoO 4 The solid was dissolved in 100 mL of distilled water to prepare 0.25M Na 2 MoO 4 solution;
[0029] 2) Pipette 200 μl o-chlorophenol with a pipette gun, and dilute it in a 250mL volumetric flask to prepare a 1g / L o-chlorophenol stock solution;
[0030] 3) Take Na 2 MoO 4 Add distilled water (a solution) to the stock solution to have a final molybdate concentration of 2 mM, and adjust the pH to 8; add o-chlorophenol to distilled water (b solution), adjust the pH to 8, and mix the two solutions;
[0031] 4) Mix a and b solutions in step 2) and wrap the reaction bottle with a black bag to make the reaction under dark reaction conditions, add hydrogen peroxide to the system at a concentration of 500 mg / L, and place the reaction system on a constant temperature shaker;
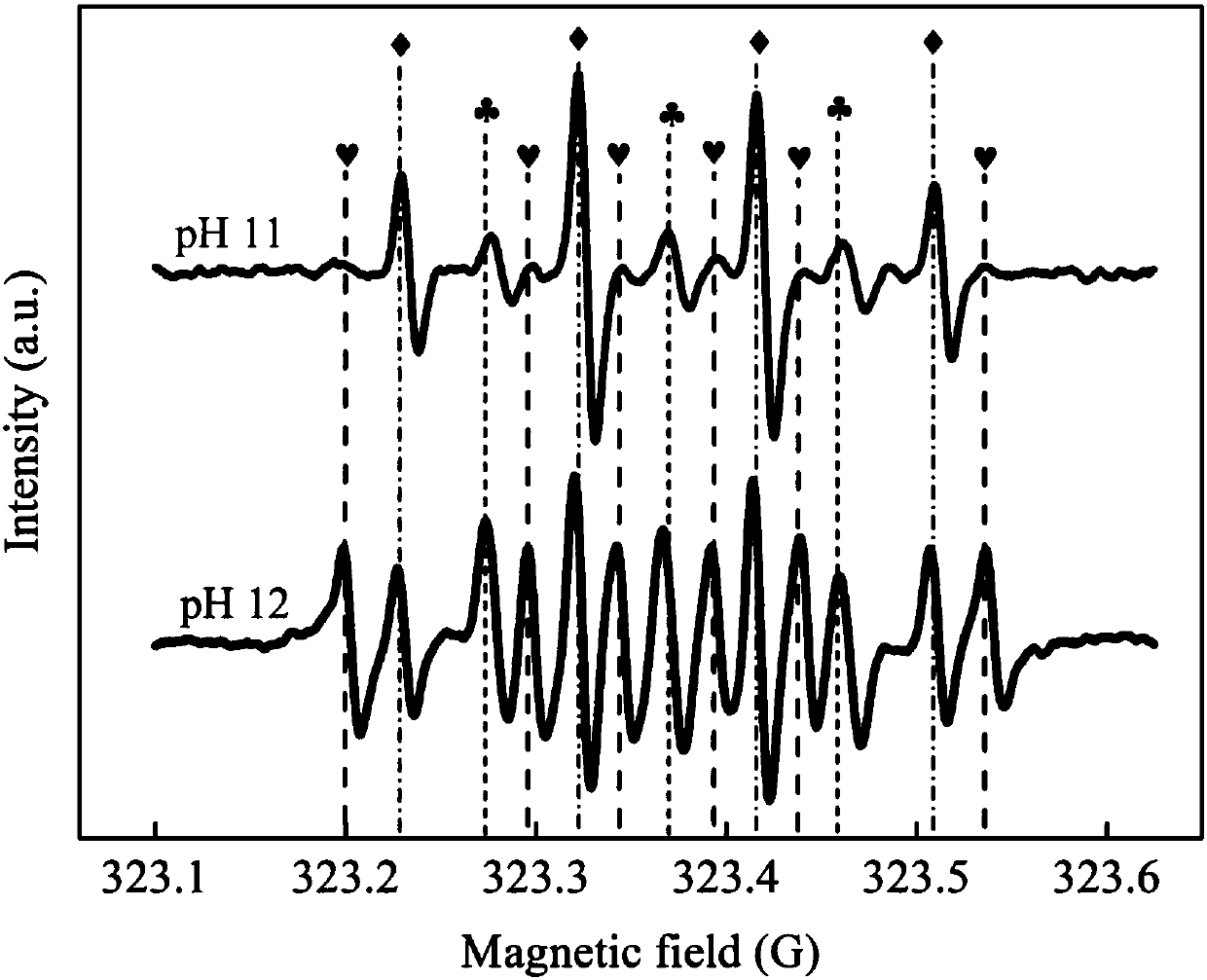
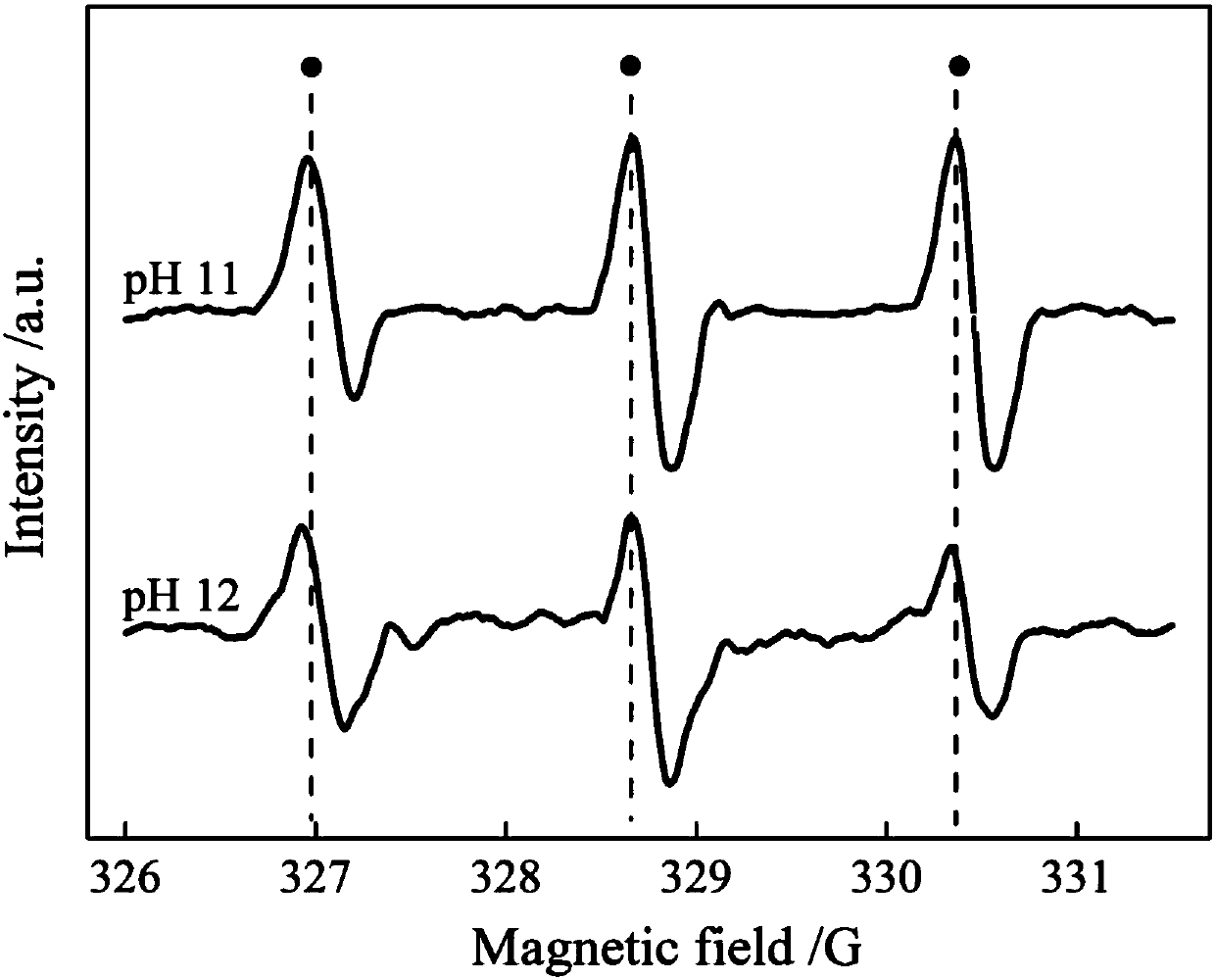
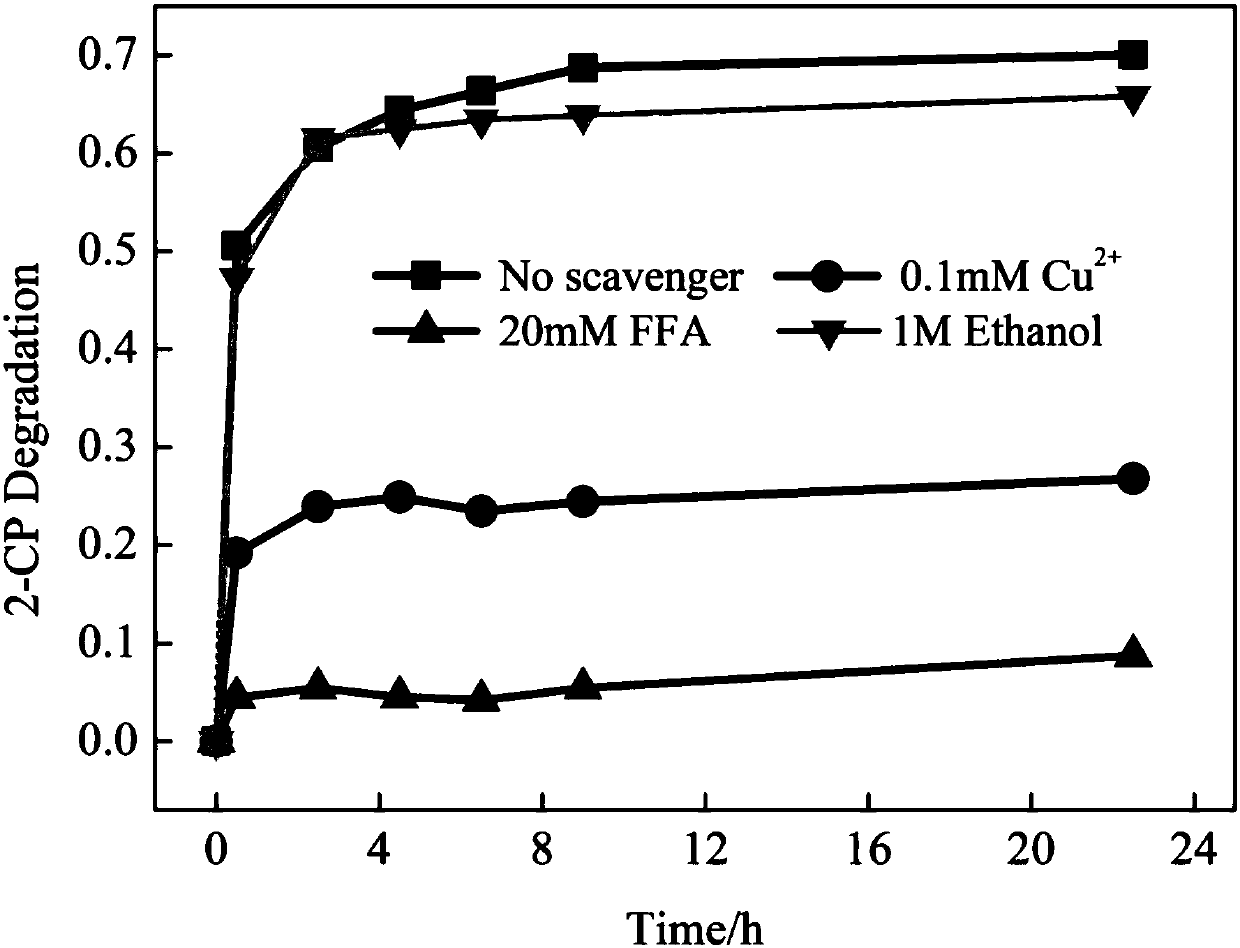
[0032] When the concentration of hydrogen peroxide is 500mg / L at pH 8, the degradation of p-chlorophenol is as follows Image 6 shown. The basic reaction reached equilib...
Embodiment 2
[0034] 1) Weigh 12.0975g of Na 2 MoO 4 Dissolve the solid in 100 mL distilled water to prepare 0.5M Na 2 MoO 4 solution;
[0035] 2) Pipette 400 μl o-chlorophenol with a pipette gun, and dilute it in a 250mL volumetric flask to prepare a 1g / L o-chlorophenol stock solution;
[0036] 3) Take Na 2 MoO 4 Add distilled water (solution a) to the stock solution to make the final concentration of molybdate 0.5mM, and adjust the pH to 11; take o-chlorophenol and add it to distilled water (solution b), adjust the pH to 11, and mix the two solutions;
[0037] 4) Mix a and b solutions in step 2) and wrap the reaction bottle with a black bag to make the reaction under dark reaction conditions, add hydrogen peroxide to the system at a concentration of 1000 mg / L, and place the reaction system on a constant temperature shaker;
[0038] When the hydrogen peroxide concentration rises to 1000mg / L at pH 11, the degradation of p-chlorophenol is as follows Image 6 shown. After 8 hours, the...
Embodiment 3
[0040] 1) Weigh 6.04875g of Na 2 MoO 4 The solid was dissolved in 100 mL of distilled water to prepare 0.25M Na 2 MoO 4 solution;
[0041] 2) Pipette 200 μl o-chlorophenol with a pipette gun, and dilute it in a 250mL volumetric flask to prepare a 1g / L o-chlorophenol stock solution;
[0042] 3) Take Na 2 MoO 4 The stock solution was added to distilled water (a solution) with a final molybdate concentration of 1 mM, and the pH was adjusted to 12; the o-chlorophenol stock solution was added to distilled water (b solution), and the pH was adjusted to 12, and the two solutions were mixed;
[0043] 4) Mix a and b solutions in step 2) and wrap the reaction bottle with a black bag to make the reaction under dark reaction conditions, add hydrogen peroxide to the system at a concentration of 1000 mg / L, and place the reaction system on a constant temperature shaker;
[0044] When the concentration of hydrogen peroxide is 1000mg / L at pH 12, the degradation of o-chlorophenol is as fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com