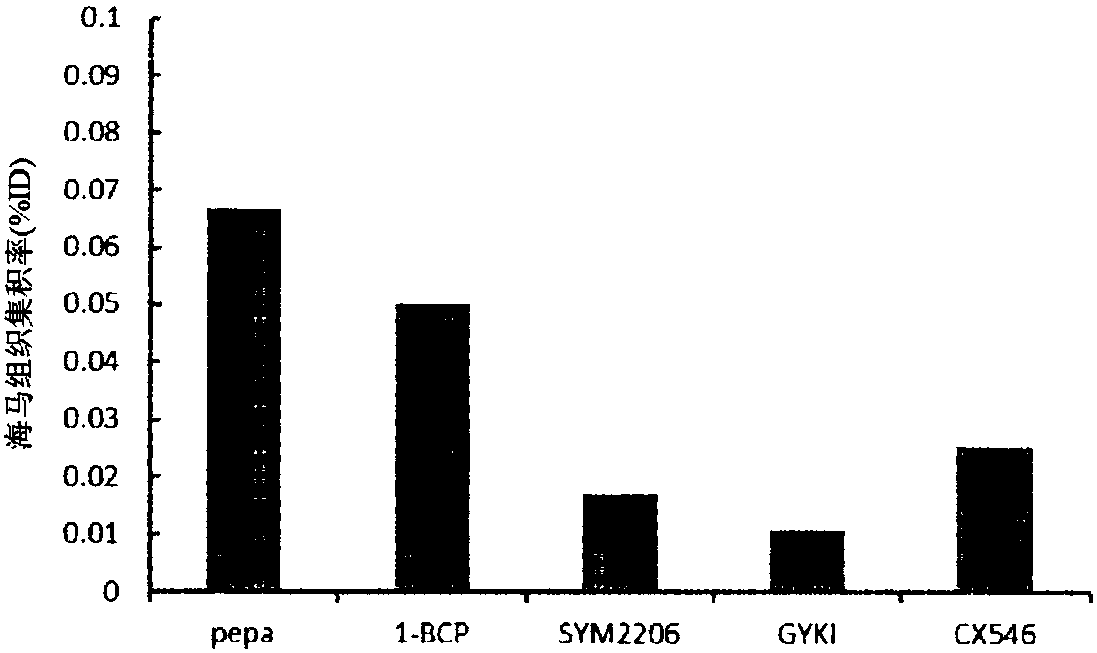
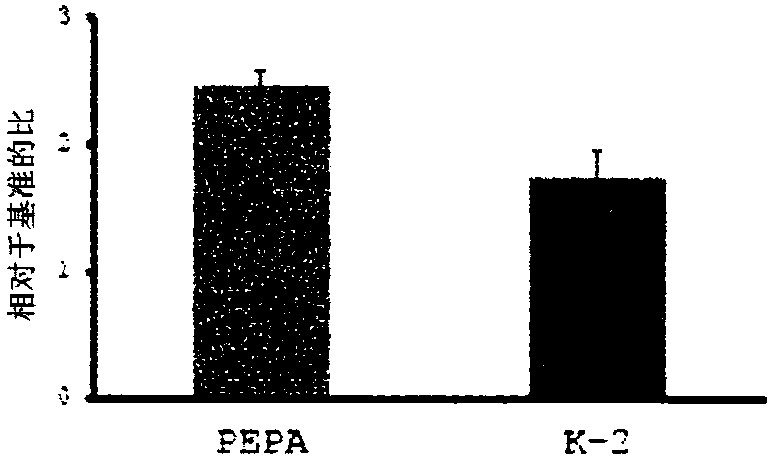
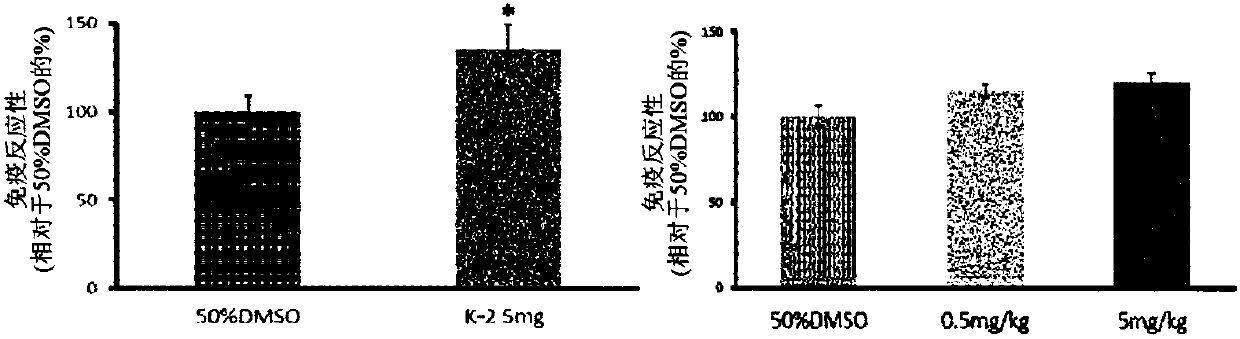
Novel compound that specifically binds to ampa receptor
一种化合物、溶剂合物的技术,应用在新颖化合物领域,能够解决探针脑移行性低、AMPA受体特异性结合不充分等问题,达到高产率、容易合成的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0082] R 2 A compound of formula (I) or a pharmaceutically acceptable salt or a solvate thereof, which is an alkyl, alkenyl or alkynyl group, can be obtained, for example, by making a compound of the following formula (II) or a pharmaceutically acceptable salt or a solvent thereof compound:
[0083] [chemical 4]
[0084]
[0085] (where A, X, Y, Z, R 1 , R 3 , R 4 , R 5 , and n are the same as defined in the compound of formula (I)) and X 1 -R 2 (where, R 2 is alkyl, alkenyl or alkynyl, X 1 It is produced by reaction of halogen). In one embodiment, R in formula (I) and formula (II) 3 and R 4 Both are hydrogen. In one embodiment, R 2 for[ 11 C] alkyl, [ 11 C] alkenyl, or [ 11 C] alkynyl, preferably R 2 for[ 11 C] alkyl especially 11 CH 3 . In one embodiment, X 1 for I. If the specific example of the compound of formula (II) is listed, there are 2-[2,6-difluoro-4-({2-[(benzenesulfonyl)amino]ethyl}thio)phenoxy]acetamide (PEPA).
[0086] The reaction ca...
Synthetic example 2
[0091] R 1 A compound of formula (I) that is an alkyl, alkenyl, or alkynyl group, or a pharmaceutically acceptable salt or solvate thereof, can be obtained by making a compound of the following formula (III), or a pharmaceutically acceptable salt thereof Salt or solvate:
[0092] [chemical 5]
[0093]
[0094] (where A, X, Y, Z, R 2 , R 3 , R 4 , R 5 , and n are the same as defined above, R a independently, are alkyl, alkenyl, or alkynyl) and X 1 -R 1 (where, R 1 Same as defined above, X 1 It is produced by reaction of halogen). In one embodiment, R a All are n-butyl. In one embodiment, R 1 for[ 11 C] alkyl, [ 11 C] alkenyl, or [ 11 C] alkynyl, preferably R 1 for[ 11 C] alkyl especially 11 CH 3 . In one embodiment, X 1 for I.
[0095] Specific examples of the compound of formula (III) are as follows.
[0096] [table 3]
[0097]
[0098] The reaction can be carried out in the presence of palladium catalysts, phosphine ligands, carbonates and coppe...
Embodiment 1
[0115] (Synthesis of K-1 and K-2)
[0116] According to the following scheme, 2-[2,6-difluoro-4-({2-[(benzenesulfonyl)amino]ethyl}thio)phenoxy]acetamide (K-1, PEPA) was synthesized and {4-[2-(phenylsulfonyl-methyl-amino)-ethylthio]-2,6-difluoro-phenoxy}-acetamide (K-2).
[0117] about each compound 1 H NMR spectrum, using TMS (tetramethylsilane, tetramethylsilane) as an internal standard, and recorded with Bruker Avance III 400MHz or Varian Mercury plus-300MHz.
[0118] [chemical 6]
[0119]
[0120] Step (i): Synthesis of (2,6-difluoro-phenoxy)-methyl acetate (2)
[0121] [chemical 7]
[0122]
[0123] To a solution of 2,6-difluoro-phenol (1) (5.00 g, 38.5 mmol) in acetone (75 mL) was added K 2 CO 3 (8.40 g, 60.7 mmol), and methyl bromoacetate (5.80 g, 38.5 mmol) was added to the reaction solution after 10 minutes. The reaction solution was stirred overnight at room temperature. After the reaction, the reaction mixture was poured into a mixture of concentrated h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com