High-performance methanol synthesis catalyst and preparation method thereof
A technology for synthesizing methanol and catalysts, applied in catalyst activation/preparation, chemical instruments and methods, preparation of hydroxyl compounds, etc., to achieve the effects of good fluidity, uniform particle size, and increased surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
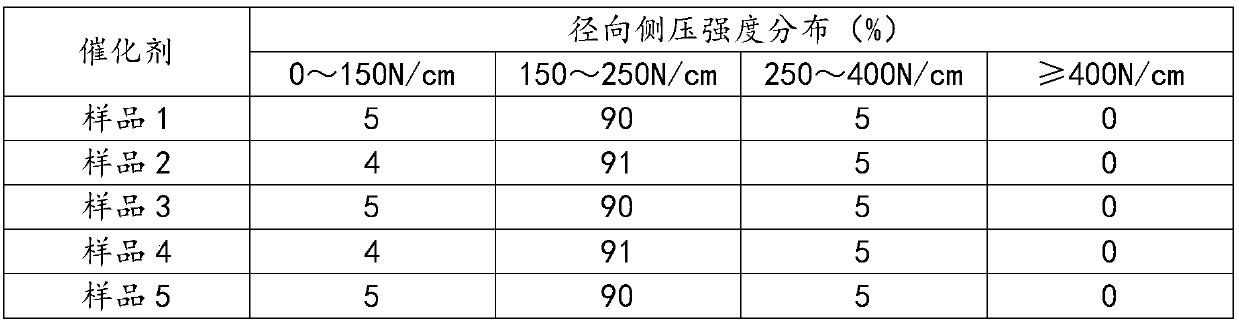
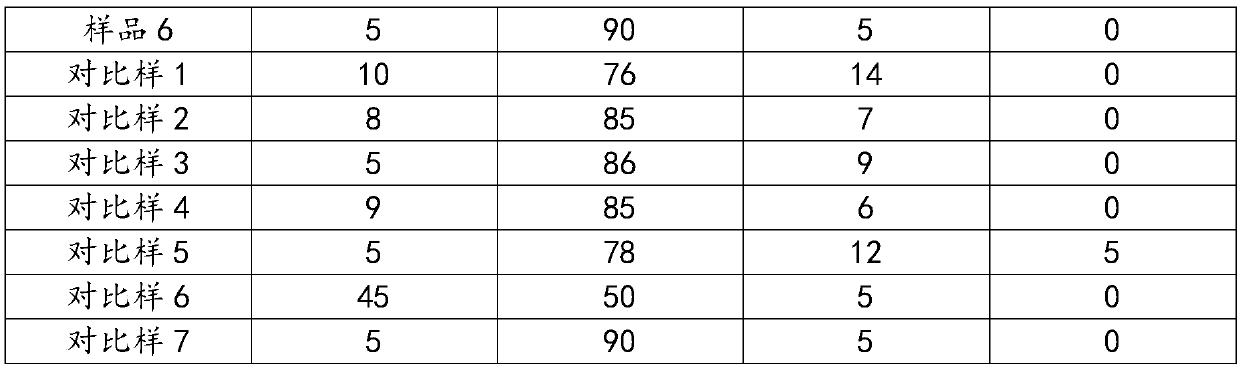
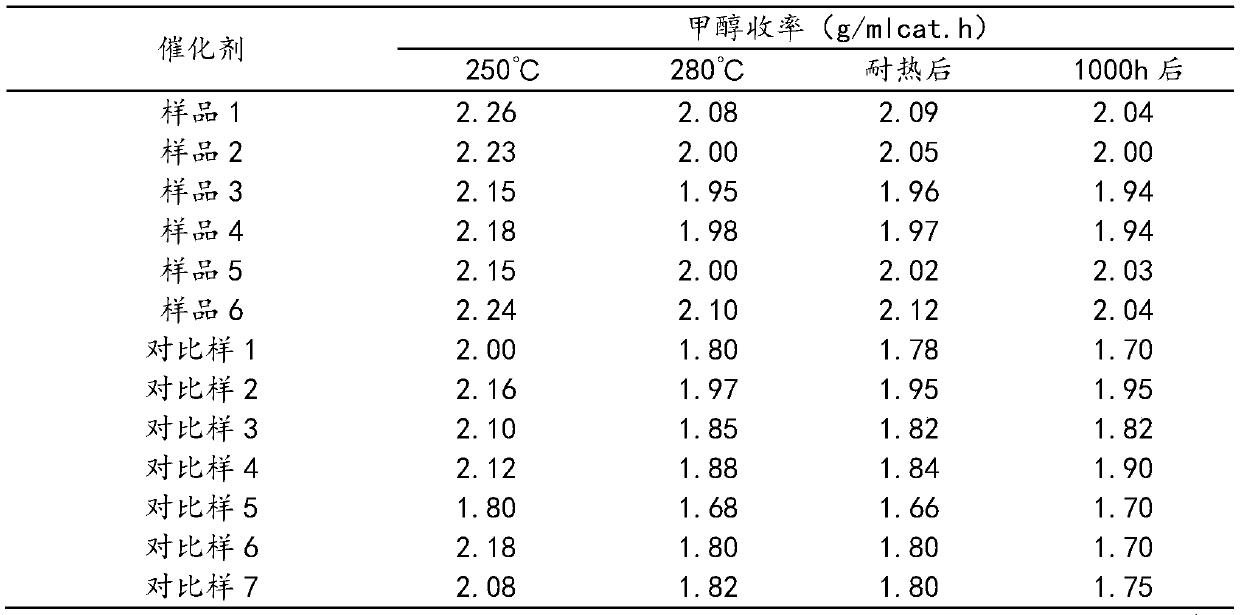
Examples
Embodiment 1
[0037] Weigh Zn(NO 3 ) 2 ·6H 2 O 758.93g, Al(NO 3 ) 2 9H 2 O 1913.93g, stirred and dissolved in a beaker, supplemented with desalted water and set the volume to 10L, and prepared salt solution A. Weigh Na 2 CO 3 1134.84g, stirred and dissolved in a beaker, supplemented with desalted water and set the volume to 10L, and prepared alkaline solution A. Weigh Cu(NO 3 ) 2 ·3H 2 O 1972.24g, Zn(NO 3 ) 2 ·6H 2 O1416.67g, Al(NO 3 ) 2 9H 2 O 255.19g was stirred and dissolved in a beaker, desalinated water was added and the volume was adjusted to 15L, and salt solution B was prepared. Weigh Na 2 CO 3 1464.60g, stirred and dissolved in a beaker, supplemented with desalted water and set the volume to 15L, and prepared alkaline solution B.
[0038] Preheat the salt solution A and alkali solution A to a reaction temperature of 80°C, while controlling the temperature of the reaction water bath to 80°C, and pour them into the reaction kettle in parallel under vigorous stirri...
Embodiment 2
[0042] Weigh Mg(NO 3 ) 2 ·6H 2 O 523.27g, Al(NO 3 ) 2 9H 2 O 1531.14g, stirred and dissolved in a beaker, supplemented with desalted water and set the volume to 7.5L, and prepared salt solution A. Weigh Na 2 CO 3 907.90g, stirred and dissolved in a beaker, supplemented with desalted water and set the volume to 7.5L, and prepared alkaline solution A. Weigh Cu(NO 3 ) 2 ·3H 2 O 1972.24g, Zn(NO 3 ) 2 ·6H 2 O934.06g, Al(NO 3 ) 2 9H 2 O 471.12g was stirred and dissolved in a beaker, supplemented with desalted water and adjusted to 8.0L, and the salt solution B was prepared. Weigh Na 2 CO 3 1280.77g, stirred and dissolved in a beaker, supplemented with desalted water and set the volume to 8.0L, and prepared alkaline solution B.
[0043] Preheat the salt solution A and alkali solution A to a reaction temperature of 75°C, while controlling the temperature of the reaction water bath to 75°C, and pour them into the reaction kettle in parallel under vigorous stirring ...
Embodiment 3
[0047] Weigh 50.0% Mn(NO 3 ) 2 ·6H 2 O 715.80g, Al(NO 3 ) 2 9H 2 O 1531.14g, stirred and dissolved in a beaker, supplemented with desalted water and set the volume to 8.0L, and prepared salt solution A. Weigh Na 2 CO 3 629.52g, stirred and dissolved in a beaker, supplemented with desalted water and set the volume to 8.0L, and prepared alkaline solution A. Weigh Cu(NO 3 ) 2 ·3H 2 O 1725.71g, Zn(NO 3 ) 2 ·6H 2 O 980.77g, Al(NO 3 ) 2 9H 2 O 206.11g was stirred and dissolved in a beaker, and desalted water was added and the volume was adjusted to 7.0L to prepare salt solution B. Weigh Na 2 CO 3 1183.0g, stirred and dissolved in a beaker, supplemented with desalted water and set the volume to 7.0L, and prepared alkaline solution B.
[0048] Preheat the salt solution A and alkali solution A to a reaction temperature of 70°C, while controlling the temperature of the reaction water bath to 70°C, and pour them into the reaction kettle in parallel under vigorous sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com