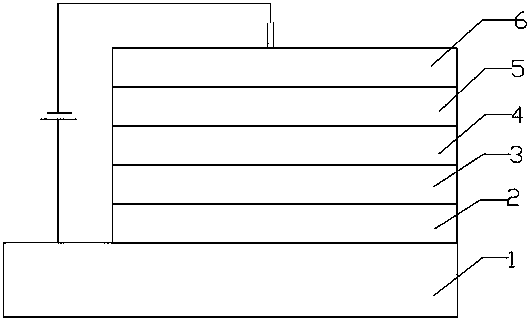
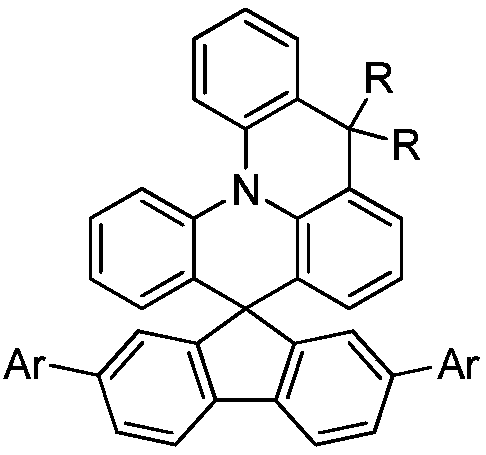
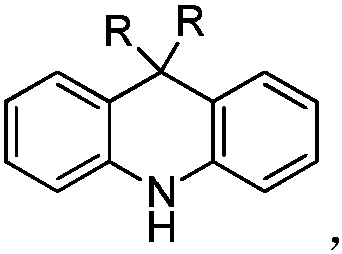
Spiro-structured organic electroluminescent composition and preparation method thereof
A spiro-ring structure and luminescent technology, applied in organic light-emitting devices, luminescent materials, organic chemistry, etc., can solve the problems of low purity of luminescent materials, difficult crystallization between molecules, slow luminous efficiency, etc., to achieve color purity, not easy to aggregate, Effect of Low Driving Voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: the preparation of compound 1:
[0054]
[0055] (1) Preparation of Intermediate I: In a 1L three-necked flask, add 9,9-dimethylacridine (41.9g, 0.20mol), o-bromoiodobenzene (62.2g, 0.22mol), sodium tert-butoxide ( 28.8g, 0.30mol), palladium acetate (0.45g, 2.0mmol), P(t-Bu) 3 HBF 4 (1.16g, 4mmol) and 500mL xylene, reacted at 120-130°C for 12h, after the reaction was over, 100g of water was added to the reaction system to quench the reaction, filtered, the filtrate was decompressed and desolvated, purified by column chromatography, and then purified by toluene, Petroleum ether was recrystallized to obtain Intermediate I with a yield of 82.37%;
[0056] (2) Preparation of intermediate II: Add the above-mentioned intermediate I (54.6g, 0.15mol) into a 1L three-necked flask, add 200g of tetrahydrofuran, cool down to -78°C, add dropwise a n-hexane solution of n-butyllithium (0.16 mol), the dropwise addition was completed, and the reaction was carried out...
Embodiment 2
[0059] Embodiment 2: the preparation of compound 2:
[0060]
[0061] Referring to Example 1, in the preparation process, the raw material 9,9-dimethylacridine was replaced with 9,9-diphenylacridine to obtain compound 2 with a yield of 45.47%.
[0062] High resolution mass spectrometry, molecular formula C 44 h 27 Br 2 N, theoretical value 727.0510, test value 727.0522.
Embodiment 3
[0063] Embodiment 3: the preparation of compound C01:
[0064]
[0065] In a 250ml three-necked flask, under nitrogen protection, compound 1 (6.03g, 0.01mol), phenylboronic acid (2.68g, 0.022mol), potassium carbonate (5.52g, 0.04mol), tetrakistriphenylphosphine palladium ( 232mg, 0.2mmol), 12mL water and 60mL toluene, reacted at 80-85°C for 10h, after the reaction was completed, filtered, the filtrate was desolvated under reduced pressure, purified by column chromatography, and then recrystallized by toluene and petroleum ether to obtain the target product C01. The rate is 83.28%.
[0066] High resolution mass spectrometry, molecular formula C 46 h 33 N, theoretical value 599.2613, test value 599.2627.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com