Arylamine derivative, and preparation method and application thereof
A technology of derivatives and aromatic amines, applied in the field of organic electroluminescent devices, to achieve low driving voltage, suitable thermal stability, and improve performance parameters
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] The application also provides the preparation method of the arylamine derivative, specifically:
[0068] reacting the compound having the structure of formula (IV) with the compound having the structure of formula (V) under the action of a catalyst and an alkali metal salt to obtain an arylamine derivative having the structure of formula (I);
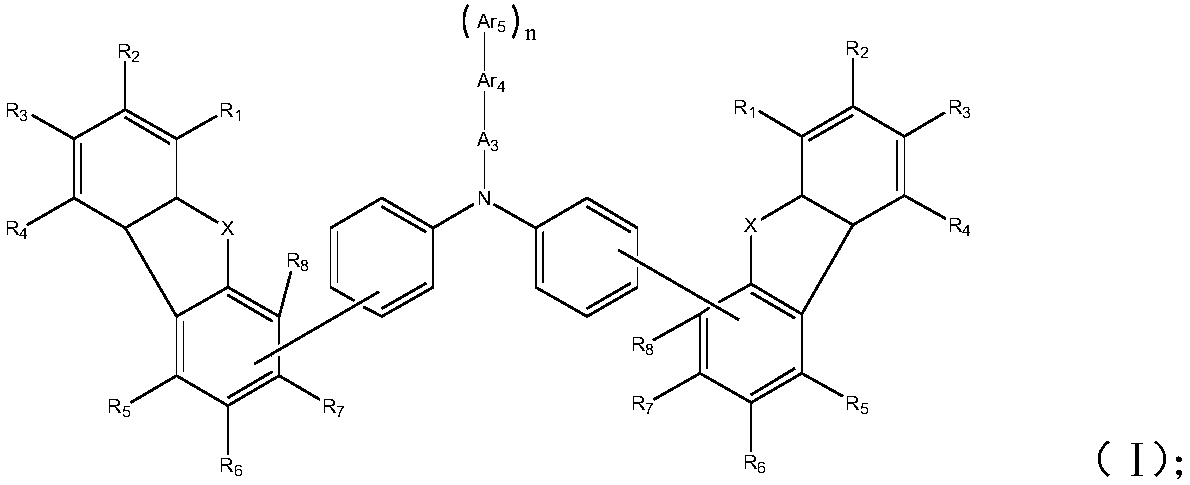
[0069]
[0070] Wherein, X is selected from N-Ar 1 , O or S;
[0071] R 1 ~R 8 Each independently selected from hydrogen, deuterium, halogen, cyano, hydroxyl, nitro, substituted or unsubstituted C1~C20 alkyl, substituted or unsubstituted C6~C30 aryl, substituted or unsubstituted C2~ C30 heteroaryl, substituted or unsubstituted C1-C20 alkoxy, substituted or unsubstituted C6-C20 aryloxy, substituted or unsubstituted C3-C40 silyloxy, substituted or unsubstituted C1-C20 acyl, substituted or unsubstituted C2-C20 alkoxycarbonyl, substituted or unsubstituted C2-C20 acyloxy, substituted or unsubstituted C2-C20 amido, substituted or ...
Embodiment 1
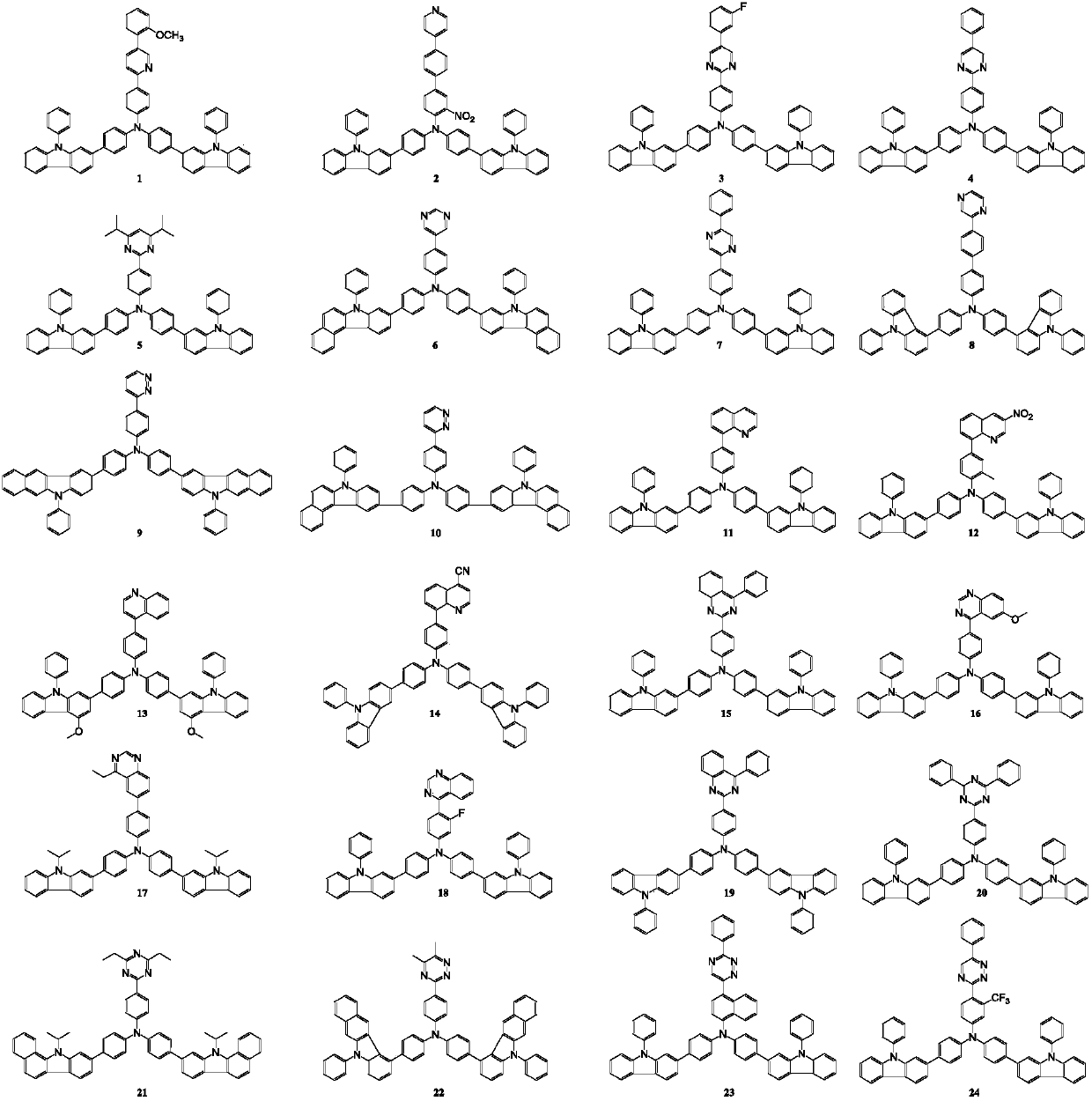
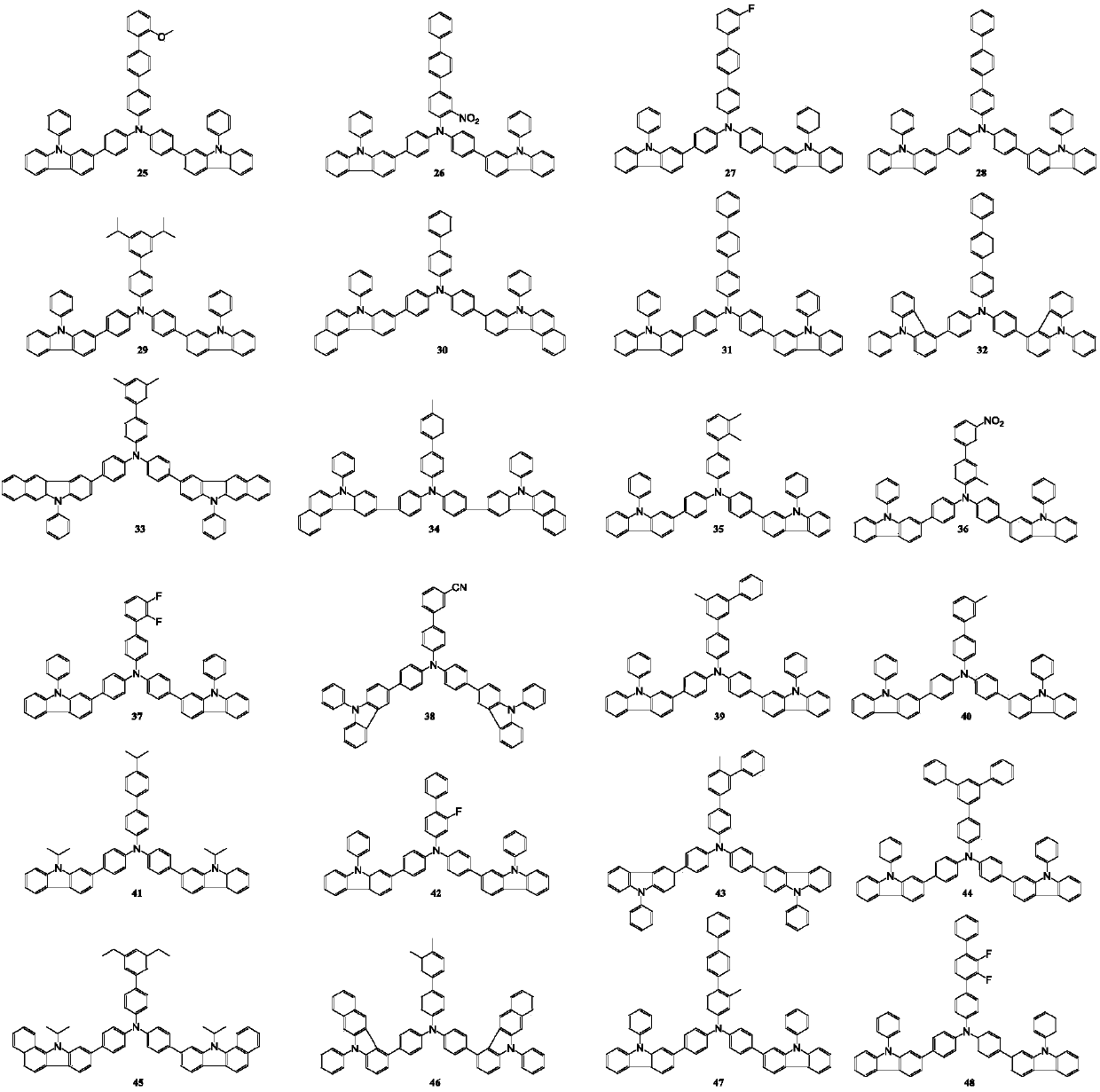
[0089] In a nitrogen atmosphere, 10.0g (11.7mmol) of intermediate M-3-1-1, 3.38g (14.04mmol) of 2-chloro-4-phenylquinazoline and 0.135g (0.12mmol) of (Triphenylphosphine) palladium is put into the flask and dissolved in the mixed solvent of 250ml of toluene, ethanol and water (volume ratio 3:1:1); then, the aqueous solution of potassium carbonate of 6g (43.6mmol) is added to the above reaction After the reaction, the reactant was extracted with ethyl acetate, and the extracted product was dried with magnesium sulfite and filtered; then, the filtered product was concentrated under reduced pressure, and silica gel column chromatography (developing agent ratio: Petroleum ether / dichloromethane=3:1) purified the concentrated product to obtain 9.38 g of compound 15 (yield=86%), mass spectrum: theoretical value 931.37, measured value 931.35. Above-mentioned reaction process is specifically as follows:
[0090]
Embodiment 2
[0092] The preparation method is the same as in Example 1, except that M-3-1-1 is replaced by M-3-1-2, 2-chloro-4-phenylquinazoline is replaced by 4-bromobiphenyl, and finally 8.55 g of compound 31 was obtained (yield=83%), mass spectrum: theoretical value 880.36, measured value 880.34. The reaction process is specifically as follows:
[0093]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com