Preparation method of polyurethane elastomer containing polylactic acid stereoisomerism composite crystal
A technology of stereocomposite crystallization and polyurethane elastomer, applied in the field of organic synthesis, can solve the problems of affecting the reproducibility of the reaction stability between batches of product performance, increase the viscosity of the reaction system, etc., to improve elasticity, improve comprehensive performance, Simple to use effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
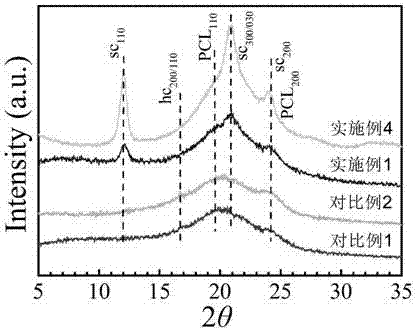
Image
Examples
Embodiment 1
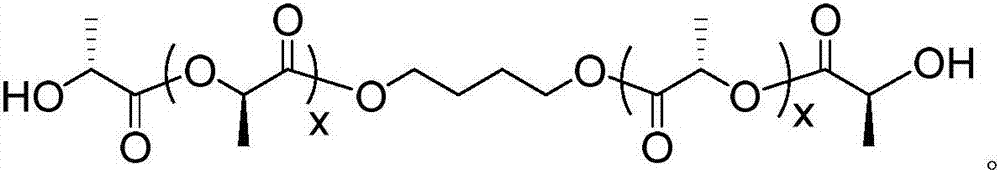
[0033] The preparation method of the polyurethane elastomer containing polylactic acid stereocomplex crystal in this embodiment is as follows:
[0034] 1. In the glove box, add dry L-lactide, 1,4-butanediol and stannous octoate into the reactor, and react at 120°C for 12 hours under the protection of nitrogen atmosphere; after the reaction, dissolve the product with chloroform , and then added to methanol at 10°C that is 10 times the mass of chloroform, suction filtered after precipitation, and vacuum-dried at 60°C for 24 hours to obtain L-polylactic acid diol with a molecular weight of 2000g / mol; the molar weight of stannous octoate is L-propyl 0.1% of the molar weight of lactide.
[0035] 2. In the glove box, add dry D-lactide, 1,4-butanediol and stannous octoate into the reactor, and react at 120°C for 12 hours under the protection of nitrogen atmosphere; Dissolved, then added to methanol at 10°C with 10 times the mass of chloroform, suction filtered after precipitation, a...
Embodiment 2
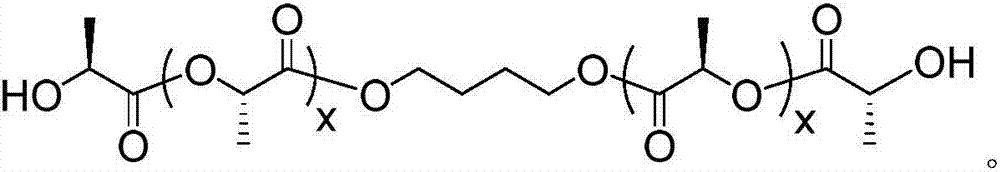
[0044] The preparation method of the polyurethane elastomer containing polylactic acid stereocomplex crystal in this embodiment is as follows:
[0045] 1. In the glove box, add dry L-lactide, 1,4-butanediol and stannous octoate into the reactor, and react at 120°C for 12 hours under the protection of nitrogen atmosphere; after the reaction, dissolve the product with chloroform , and then added to methanol at 10°C that is 10 times the mass of chloroform, suction filtered after precipitation, and vacuum-dried at 60°C for 24 hours to obtain L-polylactic acid diol with a molecular weight of 2000g / mol; the molar weight of stannous octoate is L-propyl 0.1% of the molar weight of lactide.
[0046] 2. In the glove box, add dry D-lactide, 1,4-butanediol and stannous octoate into the reactor, and react at 120°C for 12 hours under the protection of nitrogen atmosphere; Dissolved, then added to methanol at 10°C with 10 times the mass of chloroform, suction filtered after precipitation, a...
Embodiment 3
[0055] The preparation method of the polyurethane elastomer containing polylactic acid stereocomplex crystal in this embodiment is as follows:
[0056] Steps 1-4 refer to the preparation process of Example 1.
[0057] 5. Dissolve the polyurethane containing L-polylactic acid obtained in step 3 and the polyurethane containing D-polylactic acid obtained in step 4 in 30 times the volume of chloroform in a mass ratio of 4:1, stir at room temperature for 3 hours and then pour Put it into a polytetrafluoroethylene mold, let the solvent volatilize naturally, and dry it in a vacuum oven at 40°C for 24 hours to obtain a polyurethane elastomer containing polylactic acid stereocomplex crystals.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com