Chiral aluminum complex containing acetylacetone derivative and preparation method and application thereof
A technology of acetylacetone and aluminum complexes, which is applied in the field of chiral aluminum complexes and their preparation, can solve the problems of polluting the ecological environment, slow degradation, and restricting rapid development, and achieves high catalytic activity, diverse structural changes, and controllable molecular weight. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
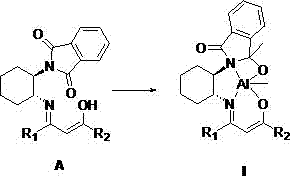
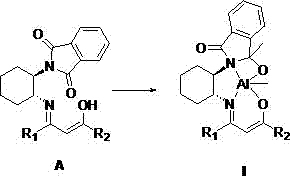
[0046] The structural formula of the synthesized ligand is the above formula (A), where R 1 is methyl; R 2 is methyl, the reaction process is: add 0.25 g of unilaterally protected chiral cyclohexanediamine (a) and equimolar amount of acetylacetone into 20 mL of methanol, heat and reflux for 10 hours, cool and filter after the reaction, and use cold washed with methanol, filtered, collected, dried and weighed to obtain 0.29 g of solid, with a yield of 87.9%.
[0047] The NMR information of the obtained product is as follows, as can be seen from the NMR information, R 1 is methyl; R 2 The ligand for the methyl group was successfully synthesized.
[0048] 1 H NMR (400 MHz, CDCl 3 ) δ 11.10 (s, 1H, O H ), 8.13 (d, J = 7.0Hz, 2H,Ar– H ), 7.62 (d, J = 7.0 Hz, 2H, Ar– H ), 5.26 (s, 1H, C H ), 4.32 (m, 1H, NC H ),3.45 (m, 1H, =NC H ), 2.54 (m, 1H, C H 2 ), 2.10 (s, 6H, C H 3 ), 2.00 (m, 5H, C H 2 ),1.52 (m, 2H, C H 2 ). HRESI-MS: m / z ccld. C 19 h 22 N 2 o ...
Embodiment 2
[0050] The structural formula of the synthesized ligand is the above formula (A), where R 1 is methyl; R 2 is trifluoromethyl, the reaction process is: add 0.30 g of unilaterally protected chiral cyclohexanediamine (a) and an equimolar amount of trifluoroacetylacetone into 20 mL of methanol, heat and reflux for 12 hours, after the reaction Cool and filter and wash with cold methanol, filter, collect, dry and weigh to obtain 0.38 g of solid, with a yield of 80.9%.
[0051] The NMR information of the obtained product is as follows, as can be seen from the NMR information, R 1 is methyl; R 2 The ligand for trifluoromethyl was synthesized successfully.
[0052] 1 H NMR (400 MHz, CDCl 3 ) δ 11.03 (s, 1H, O H ), 8.17 (d, J = 7.2 Hz, 2H,Ar– H ), 7.52 (d, J = 7.2 Hz, 2H, Ar– H ), 5.70 (s, 1H, C H ), 4.33 (m, 1H, NC H ),3.45 (m, 1H, =NC H ), 2.58 (m, 1H, C H 2 ), 2.12 (s, 3H, C H 3 ), 2.04 (m, 5H, C H 2 ),1.61 (m, 2H, C H 2 ). HRESI-MS: m / z cacld.C 19 h 19 f...
Embodiment 3
[0054] The structural formula of the synthesized ligand is the above formula (A), where R 1 is trifluoromethyl; R 2 is a phenyl group, the reaction process is: add 0.20 g of unilaterally protected chiral cyclohexanediamine (a) and an equimolar amount of benzoyltrifluoroacetone to 10 mL of methanol, heat and reflux for 8 hours, and after the reaction Cool and filter and wash with cold methanol, filter, collect, dry and weigh to obtain 0.29 g of solid, with a yield of 80.6%.
[0055] The NMR information of the obtained product is as follows, as can be seen from the NMR information, R 1 is trifluoromethyl; R 2 The ligand for phenyl was synthesized successfully.
[0056] 1 H NMR (400 MHz, CDCl 3 ) δ 12.74 (s, 1H, O H ), 8.08 (d, J = 7.0 Hz, 2H,Ar– H ), 7.53 (d, J = 7.0 Hz, 2H, Ar– H ), 7.42(m, 3H, Ar– H), 7.17 (d, J = 6.2Hz, 2H, Ar– H ), 6.52 (s, 1H, C H ), 4.48 (m, 1H, NC H), 3.92 (m, 1H, =NC H 2 ), 2.57(m, 1H, C H 2 ), 2.10 (m, 5H, C H 2 ), 1.54 (m, 2H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com