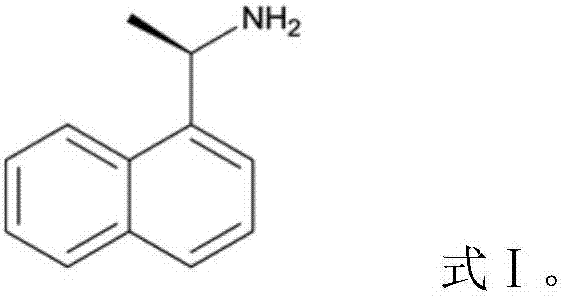
Method for detecting (R)-1-(1-naphthyl)ethylamine chiral isomer
A technology for chiral isomers and ethylamine, applied in the field of drug analysis, can solve problems such as undiscovered, and achieve the effect of simple and convenient high-performance liquid phase detection method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
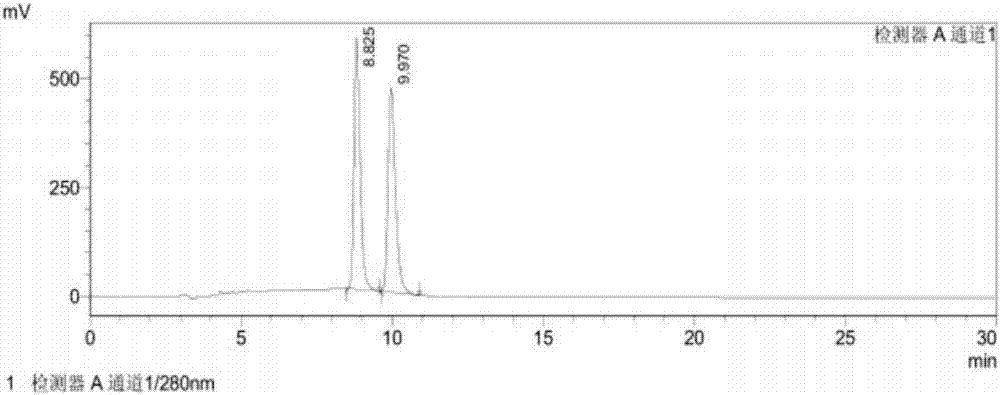
[0029] A method for detecting (R)-1-(1-naphthyl)ethylamine chiral isomers, comprising the following steps:
[0030] 1) Preparation of system suitability solution: Weigh appropriate amounts of (R)-1-(1-naphthyl)ethylamine and (S)-1-(1-naphthyl)ethylamine respectively, and place them in the same volumetric flask. Add 1ml of ethanol to dissolve, and then use the mobile phase solution to prepare a solution containing 0.5mg of (R)-1-(1-naphthyl)ethylamine and (S)-1-(1-naphthyl)ethylamine per ml , to obtain the system suitability solution;
[0031] 2) Preparation of the test solution: take an appropriate amount of the test solution, add 1ml of ethanol to dissolve, then use the mobile phase solution to prepare a solution with a concentration of 1mg / ml, and add it to the test solution;
[0032] 3) Sample injection: inject the system suitability solution and the test solution into the high performance liquid chromatograph respectively, measure according to the set high performance liq...
Embodiment 2
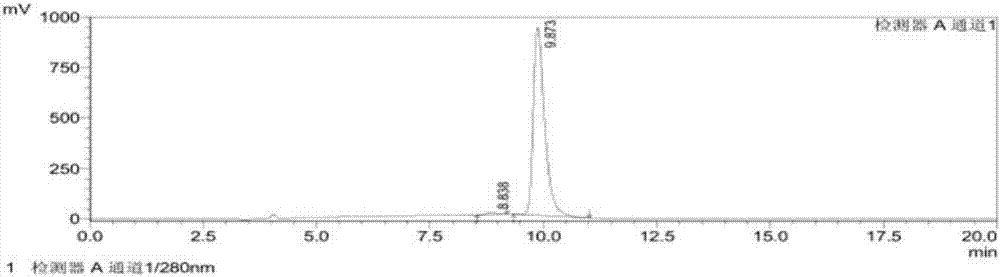
[0039] A method for detecting (R)-1-(1-naphthyl)ethylamine chiral isomers, comprising the following steps:
[0040] 1) Preparation of system suitability solution: Weigh appropriate amounts of (R)-1-(1-naphthyl)ethylamine and (S)-1-(1-naphthyl)ethylamine respectively, and place them in the same volumetric flask. Add 1ml of ethanol to dissolve, and then use the mobile phase solution to prepare a solution containing 0.5mg of (R)-1-(1-naphthyl)ethylamine and (S)-1-(1-naphthyl)ethylamine per ml , to obtain the system suitability solution;
[0041] 2) Preparation of the test solution: take an appropriate amount of the test solution, add 1ml of ethanol to dissolve, then use the mobile phase solution to prepare a solution with a concentration of 1mg / ml, and add it to the test solution;
[0042]3) Sample injection: inject the system suitability solution and the test solution into the high performance liquid chromatograph respectively, measure according to the set high performance liqu...
Embodiment 3
[0047] A method for detecting (R)-1-(1-naphthyl)ethylamine chiral isomers, comprising the following steps:
[0048] 1) Preparation of system suitability solution: Weigh appropriate amounts of (R)-1-(1-naphthyl)ethylamine and (S)-1-(1-naphthyl)ethylamine respectively, and place them in the same volumetric flask. Add 1mL of ethanol to dissolve, and then use the mobile phase solution to prepare a solution containing 0.5mg of (R)-1-(1-naphthyl)ethylamine and (S)-1-(1-naphthyl)ethylamine per ml , to obtain the system suitability solution;
[0049] 2) Preparation of the test product solution: take an appropriate amount of the test product, add 1 mL of ethanol to dissolve, then use the mobile phase solution to prepare a solution with a concentration of 1 mg / mL, and add it to the test product solution;
[0050] 3) Sample injection: inject the system suitability solution and the test solution into the high performance liquid chromatograph respectively, measure according to the set hig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com