Synthetic method for bisoprolol fumarate process impurities
A technology for bisoprolol fumarate and process impurities, which is applied in the field of chemical pharmacy to achieve the effects of cheap raw materials, improved accurate positioning and qualitative, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
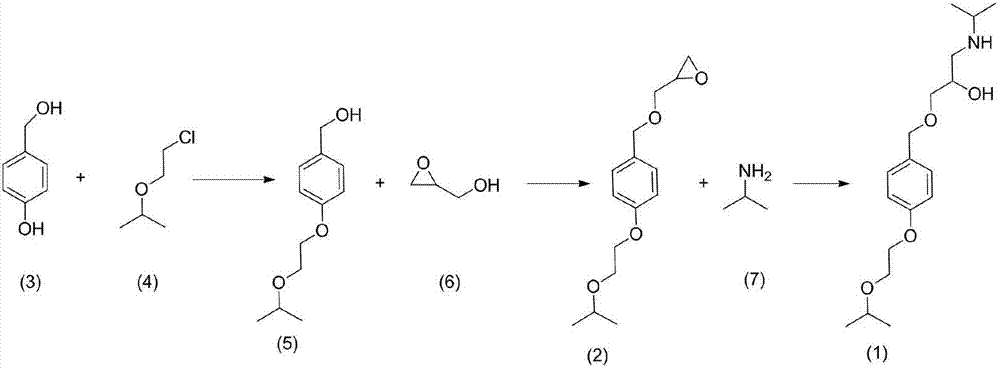
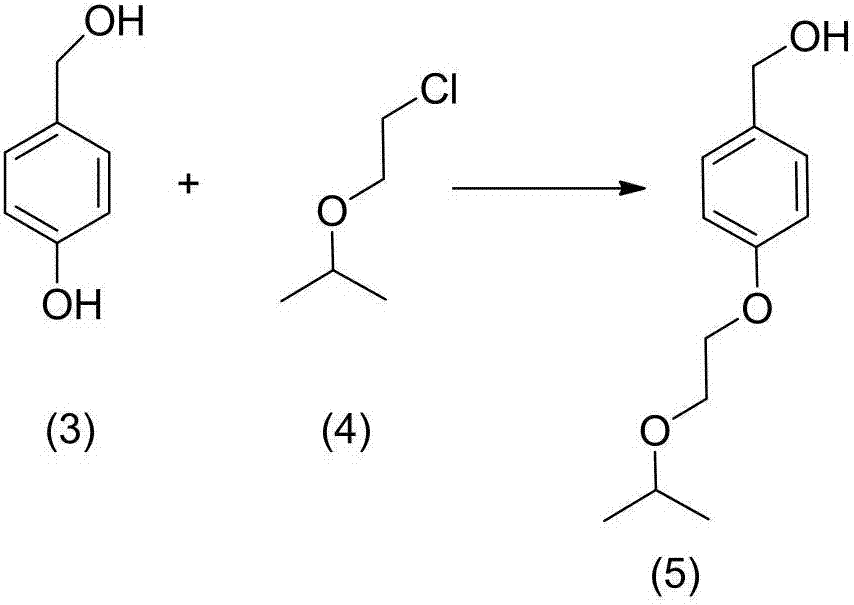
[0014] Example 1: Preparation of (4-(2-isopropoxyethoxy)phenyl)methanol (5)
[0015]
[0016] Add 4-hydroxybenzyl alcohol (100.0g, 0.805mol), 2-(2-chloroethoxy)propane (118.42g, 0.966mol), triethylamine (122.19, 1.21mol) into a 1000ml three-necked flask, and start stirring , the temperature was raised to 40° C., and detected by TLC, it was found that the raw material 4-hydroxybenzyl alcohol disappeared after 2 hours of reaction, and the reaction ended. Add 200ml of water, 400ml of dichloromethane, separate the aqueous phase and wash with 100ml of dichloromethane for 3 times, combine the organic phase, wash the organic phase with 100ml of saturated saline for 3 times, reduce the organic phase to 150ml, add silica gel and continue vacuum distillation to dryness , the residue was subjected to silica gel column chromatography and eluted with dichloromethane-methanol (80:1) to obtain 48.25 g of an oily substance, 28.51%. 1 HNMR (600MHz, DMSO-d6, δppm): 1.25(t, 6H), 3.21(m, 2H),...
Embodiment 2
[0017] Example 2: Preparation of 2-((((4-(2-isopropoxyethoxy)benzyl)oxy)methyl)oxirane (2)
[0018]
[0019] Add (4-(2-isopropoxyethoxy)phenyl)methanol (46g, 0.219mol) and 130ml methanol to a 500ml three-necked flask and stir, add p-toluenesulfonic acid (2.5g, 0.015mol), The temperature of oxirane-2-ylmethanol (201.48 g, 2.19 mol) was raised to 40° C., and the reaction was carried out for 4 h, and the reaction was detected by TLC. Add 25% NaOH solution, 200ml of water, and 600ml of dichloromethane, wash the aqueous phase with 100ml of dichloromethane for 3 times, combine the organic phases, and wash the organic phase with 100ml of saturated brine for 3 times. The organic phase was rotary evaporated to obtain 49.23 g of oil, with a yield of 84.47%. HPLC detection 98.49%.
[0020] 1 HNMR (600MHz, DMSO-d6, δppm): 1.25(t, 6H), 2.34(m, 1H), 2.61(m, 1H), 2.85(m, 1H), 3.21(m, 2H), 3.36(m, 1H), 3.60(s, 1H), 3.62(m, 1H), 3.71(t, 2H), 4.42(t, 2H), 4.72(s, 2H), 6.28(d, 2H), 6.86(d...
Embodiment 3
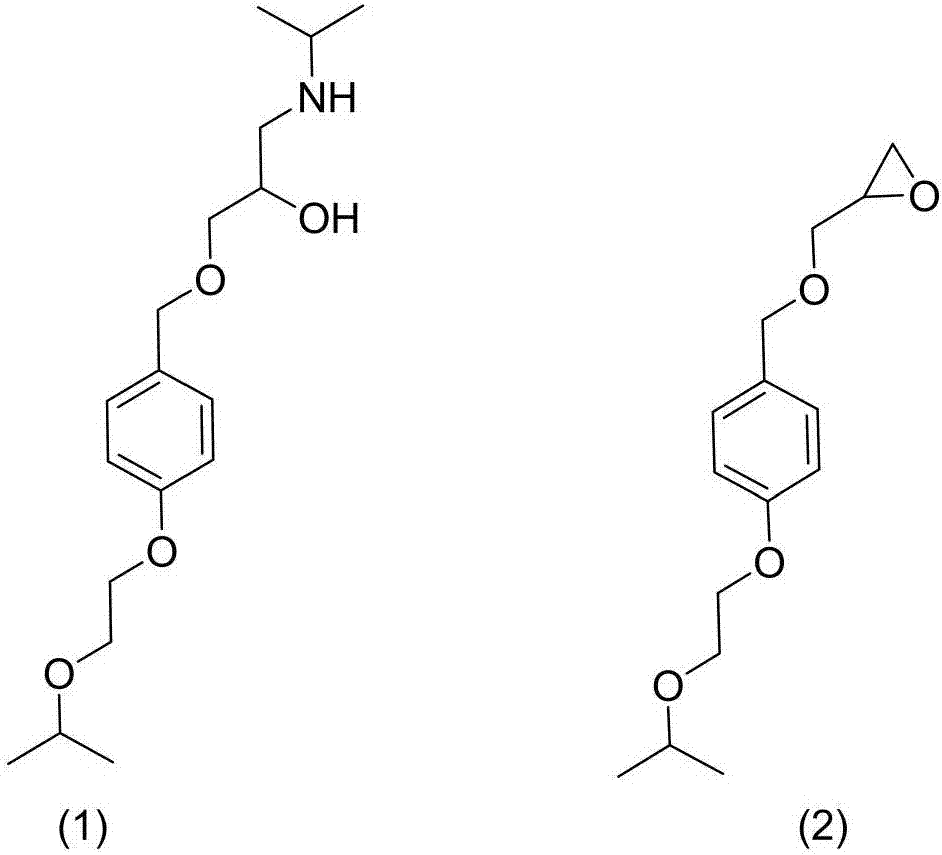
[0021] Example 3: Preparation of 1-((4-(2-isopropoxyethoxy)benzyl)oxy)-3-(isopropylamino)propan-2-ol (1)
[0022]
[0023] Add 2-((((4-(2-isopropoxyethoxy)benzyl)oxy)methyl)oxirane (45g, 0.169mol), isopropylamine (29.95g , 0.507mol), ethanol 50ml stirring, 60 ℃ of stirring 2 hours, TLC detection reaction finishes.Add 200ml water, 400ml dichloromethane, separate liquid and discard the water phase, rotary steam the organic phase to obtain oily matter 30.35g yield 76.36%, HPLC 98.66% detected.
[0024] 1 HNMR (600MHz, DMSO-d6, δppm): 1.10(t, 6H), 1.25(t, 6H), 2.04(t, 1H), 2.58(m, 1H), 2.83(m, 1H), 2.97(m, 1H), 3.21(m, 2H), 3.46(t, 2H), 3.54(s, 1H), 3.60(s, 1H), 3.71(t, 2H), 3.76(m, 1H), 4.42(t, 2H ), 4.72 (s, 2H), 6.28 (d, 2H), 6.86 (d, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com