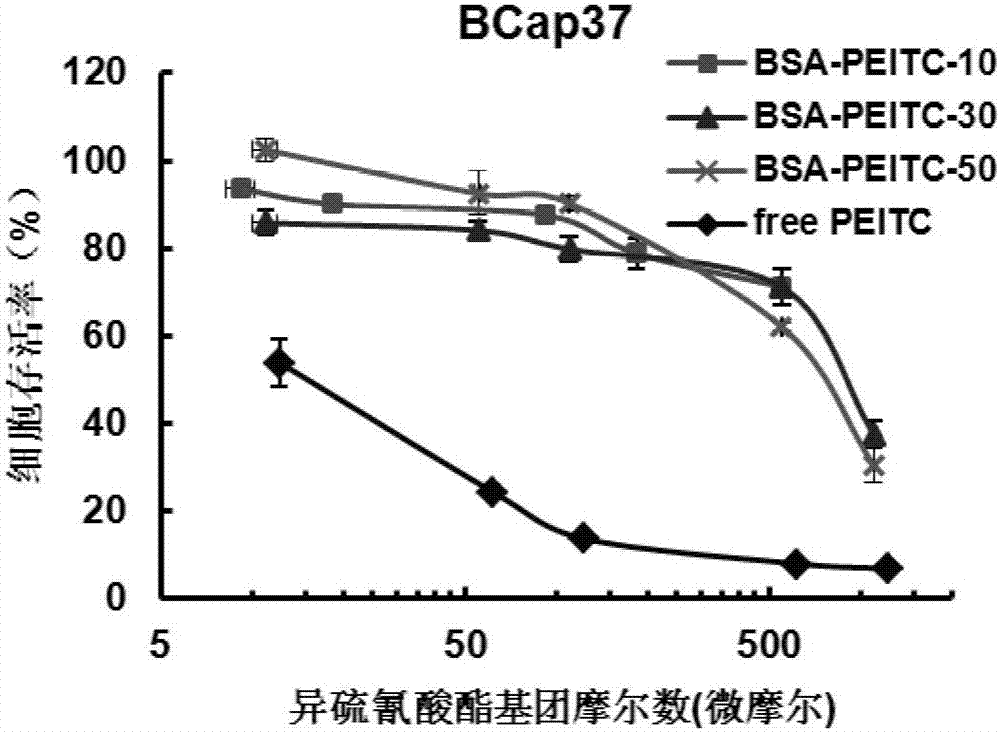
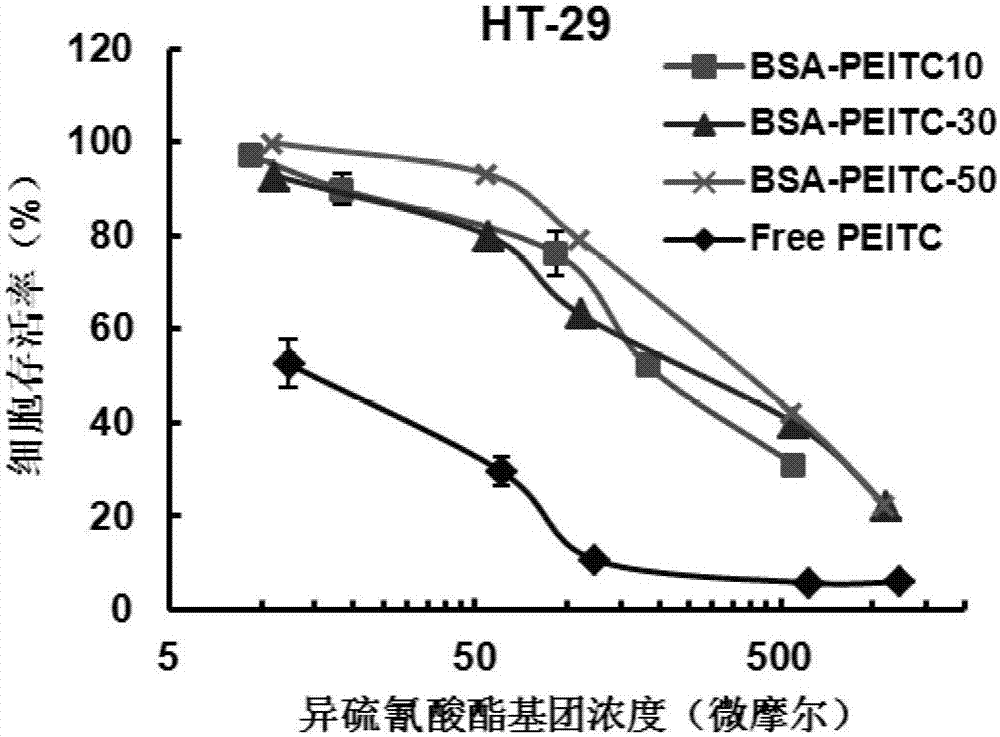
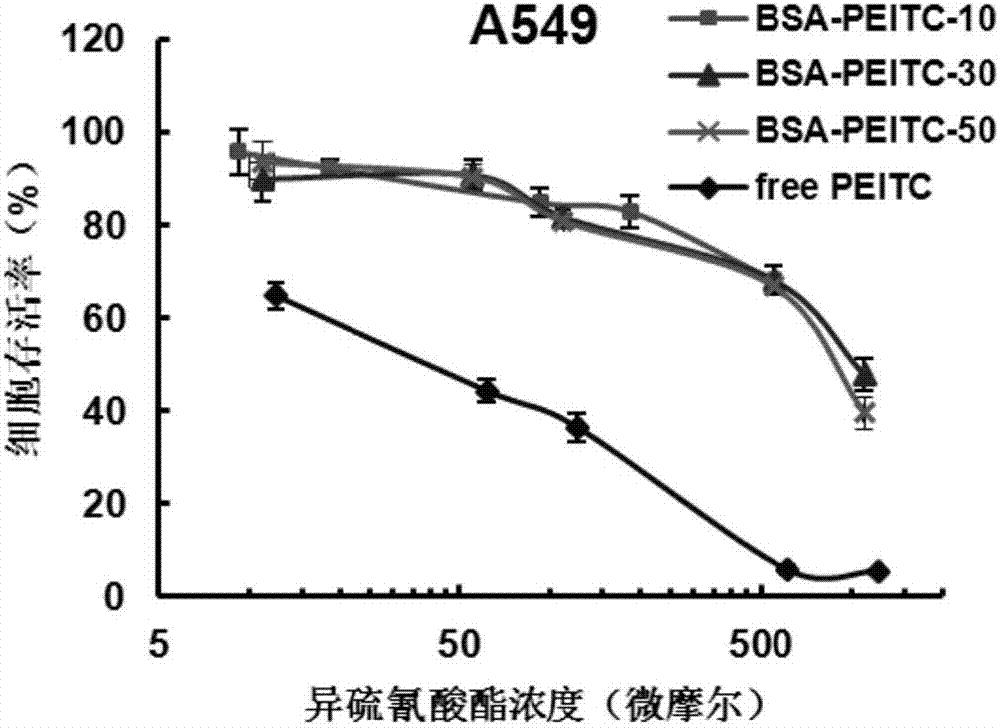
Protein-ITC (isothiocyanate) bonded substance and application thereof
A technology of isothiocyanate and isothiocyanate, which is applied in the directions of ester active ingredients, non-active ingredients of polymer compounds, drug combinations, etc., to achieve the effects of excellent water solubility, good loading and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Preparation of protein-isothiocyanate bond
[0049] a. Feeding at the molar ratio of bovine serum albumin to phenethyl isothiocyanate 1:10: Dissolve 10 mg of phenethyl isothiocyanate (PEITC, Mw = 163.24) in 4 ml of dimethyl methylene In sulfone; 410 mg of bovine serum albumin (BSA, Mw=67000) was dissolved in 20 mL of water.
[0050] b. Mix the above two solutions separately, adjust the pH to 8.5 with 1M NaOH, and react at 37°C. Take a small amount of the solution and use high performance liquid chromatography (HPLC) to monitor the progress of the reaction in real time.
[0051] c. Centrifuge at 3000rpm for 10min. The precipitate was removed by filtration, and the organic solvent was removed by dialysis to obtain a water-soluble albumin-isothiocyanate bond.
[0052] In this embodiment, the molar ratio of albumin to phenethyl isothiocyanate is 1:10, that is, an albumin theoretically connects 10 isothiocyanate groups, which is denoted as BSA-PEITC10.
Embodiment 2~3
[0054] The preparation process is exactly the same as in Example 1, except that the molar ratios of bovine serum albumin and phenethyl isothiocyanate are 1:30 and 1:50, respectively, which are denoted as BSA-PEITC30 and BSA-PEITC50, respectively.
Embodiment 4
[0056] The preparation process is exactly the same as in Example 1, except that the dimethyl sulfoxide is replaced with absolute ethanol, which is denoted as BSA-PEITC10-1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com