Preparation method of indium nitrate solution
An indium nitrate and solution technology, which is applied in chemical instruments and methods, gallium/indium/thallium compounds, inorganic chemistry, etc., can solve the problems of complex process, waste of nitric acid, and high cost of nitrogen oxides, and achieves simple preparation process and acid consumption. small amount of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
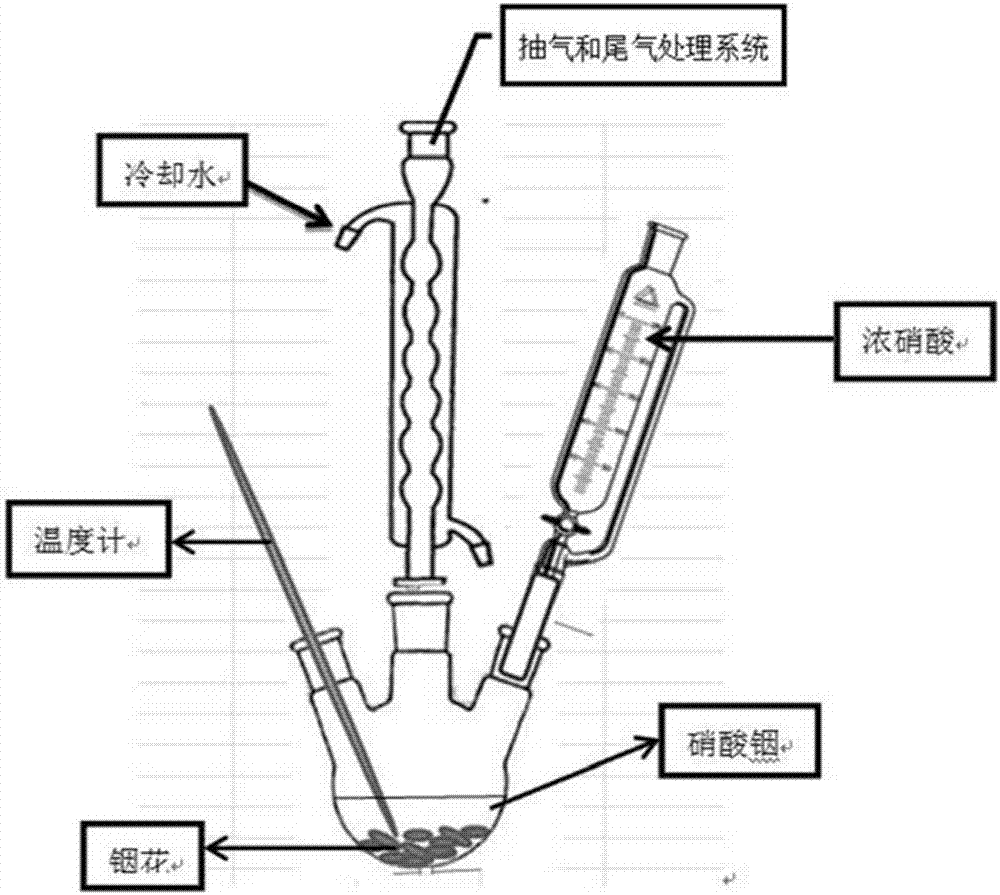
[0010] The preparation method of indium nitrate solution according to the present application includes the steps: S1, heating and melting metal indium and then water quenching to form indium flowers; S2, preparing one or more additives among oxalic acid, nitric acid, and nitrate into a solution and then adding to the reaction In the kettle, add indium flowers after heating up to 60°C, adjust the temperature of the reaction kettle to keep it constant; S3, add concentrated nitric acid to the reaction kettle for reaction, stop heating after adding concentrated nitric acid, and continue the reaction for a period of time to obtain indium nitrate solution.
[0011] In the preparation method of the present application, the metal indium is heated and melted and quenched into indium flowers with a larger specific surface area and then reacted, which can increase the contact area between the metal indium and concentrated nitric acid, and has the advantages of accelerating the reaction sp...
Embodiment 1
[0026] Heat and melt 500g metal indium and quench it with water to have a specific surface area of 4cm 2 / g~8cm 2 / g of indium flowers; configure 2L of ammonium nitrate solution with a concentration of 0.2mol / L and add it to a 5L reaction kettle, add indium flowers after heating up to 60°C, and control the temperature of the reaction kettle to 100°C during this process; put the reaction kettle Open the external exhaust device to send air into the reaction kettle, and at the same time, the tail gas generated during the reaction process is processed through the external water tank, and concentrated nitric acid is added to the reaction kettle, and the reaction temperature is controlled at 100°C. After adding 700ml of concentrated nitric acid, the acid addition is stopped. It took 6.5 hours; after stopping the heating, the reaction continued for 4 hours, and the reaction ended.
[0027] It can be seen from the test that the average production of nitrogen oxides during the react...
Embodiment 2
[0029] Heat and melt 500g metal indium and quench it with water to have a specific surface area of 4cm 2 / g~8cm 2 / g of indium flowers; configure 2L of ammonium nitrate solution with a concentration of 0.8mol / L and add it to a 5L reaction kettle, add indium flowers after heating up to 60°C, and control the temperature of the reaction kettle to 100°C during this process; put the reaction kettle Open the external exhaust device to send air into the reaction kettle, and at the same time, the tail gas generated during the reaction process is processed through the external water tank, and concentrated nitric acid is added to the reaction kettle, and the reaction temperature is controlled at 100°C. After adding 700ml of concentrated nitric acid, the acid addition is stopped. It took 6.5 hours; after stopping the heating, the reaction continued for 6 hours, and the reaction ended.
[0030] It can be seen from the test that the average production amount of nitrogen oxides during th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com