A kind of synthetic method of hexafluoro-1-butanol
A synthetic method and technology of butanol, applied in the direction of organic chemistry, oxygen-containing functional group reduction preparation, etc., can solve the problem of not being able to obtain a single product, and achieve the effects of mature reaction technology, conventional raw materials, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
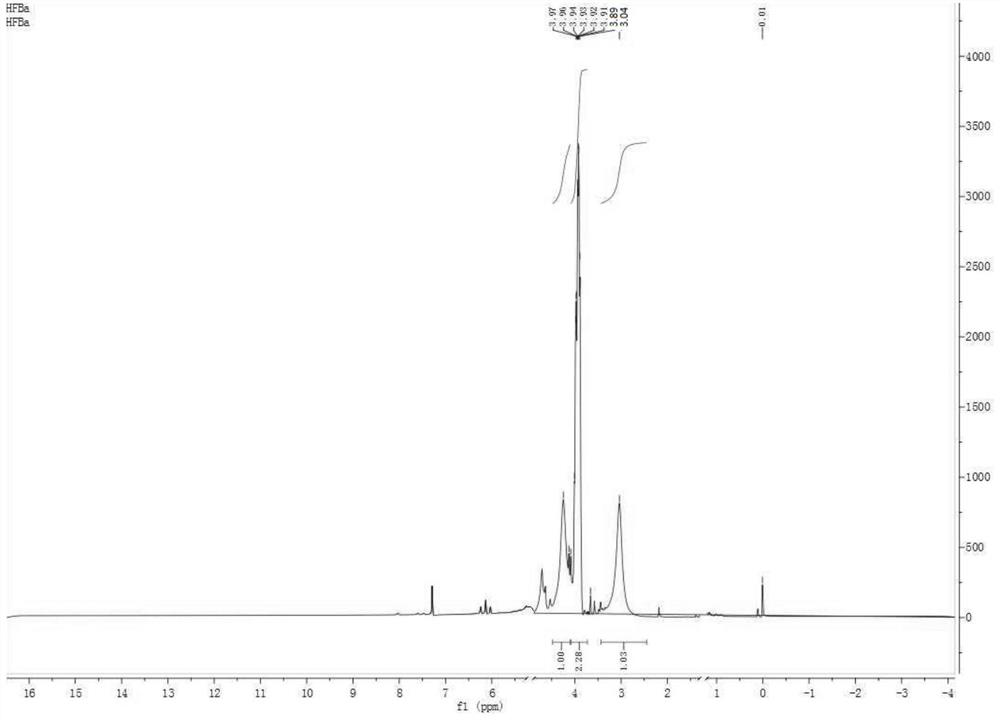
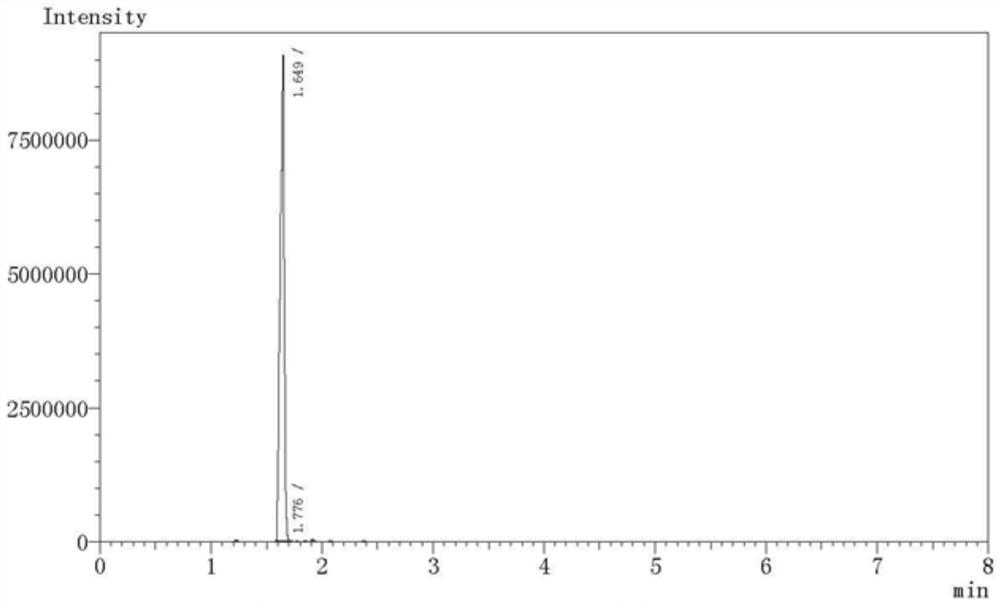
Image
Examples
preparation example Construction
[0030] The invention provides a synthetic method of hexafluoro-1-butanol, comprising:
[0031] The compound of the formula (I) and hydrogen undergo an addition reaction under the catalysis of a noble metal catalyst to obtain hexafluoro-1-butanol;
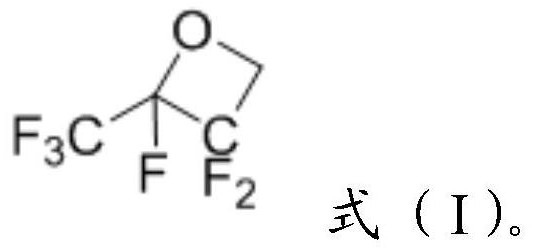
[0032]
[0033] The compound of the formula (I) structure provided by the present invention is preferably prepared according to the method of the present invention:
[0034] Hexafluoropropylene reacts with formaldehyde to obtain a compound of formula (I).
[0035] More preferably, hexafluoropropylene and formaldehyde undergo addition reaction in a solvent to prepare the compound of formula (I).
[0036] The present invention does not limit the sources of the hexafluoropropylene and formaldehyde, which can be commercially available.
[0037] According to the present invention, the molar ratio of the hexafluoropropylene to formaldehyde is preferably (1-6):1; more preferably (1-3):1; and most preferably 3:1.
[0038] The reaction...
Embodiment 1
[0062] Add 60.0g of hexafluoropropylene and 50g of hydrogen fluoride into a 500ml reaction kettle, mix well under mechanical stirring, then raise the temperature of the reaction bottle to 50°C, slowly inject 12g of formaldehyde into the reaction kettle with a metering pump, after the feeding of formaldehyde is completed, continue React for 12 hours, then stop the reaction, remove hydrogen fluoride by distillation, neutralize the crude product with 10% sodium hydroxide solution to a pH value of 7-8, separate phases, dry the organic phase with anhydrous sodium sulfate, and then carry out rectification to the crude product After purification, 64.8 g of 1-trifluoromethyl-trifluorooxetane was obtained with a yield of 90%.
[0063] The obtained 1-trifluoromethyl-trifluorooxetane 64.8g is subjected to hydrogenation hydrogenation. First, the reactor is evacuated and replaced with nitrogen for three times, and the air in the reactor is completely replaced, and then 1g of palladium carbo...
Embodiment 2
[0066] Add 60.0g of hexafluoropropylene and 50g of hydrogen fluoride into a 500ml reaction kettle, mix well under mechanical stirring, then raise the temperature of the reaction bottle to 50°C, slowly inject 12g of formaldehyde into the reaction kettle with a metering pump, after the feeding of formaldehyde is completed, continue React for 12 hours, then stop the reaction, remove hydrogen fluoride by distillation, neutralize the crude product with 10% sodium hydroxide solution to a pH value of 7-8, separate phases, dry the organic phase with anhydrous sodium sulfate, and then carry out rectification to the crude product After purification, 64.8 g of 1-trifluoromethyl-trifluorooxetane was obtained with a yield of 90%.
[0067] The obtained 1-trifluoromethyl-trifluorooxetane 64.8g is subjected to hydrogenation hydrogenation. First, the reactor is evacuated and replaced with nitrogen for three times, and the air in the reactor is completely replaced, and then 1g of platinum carbon...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com