A kind of preparation method of trifluoromethyl (trimethyl) silane
A technology of trimethylsilane and trifluoromethyl, applied in the field of preparation of trifluoromethylsilane, can solve the problems of complex operation, high equipment requirements, lack of effectiveness, etc., and achieve the effects of mild reaction conditions and conventional raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The invention provides a kind of preparation method of trifluoromethyl (trimethyl) silane, comprising:
[0021] Trifluoromethanesulfonyl chloride and trimethylsilane initiate a free radical coupling reaction under the action of light or a free radical initiator to obtain trifluoromethyl (trimethyl) silane.
[0022] In the present invention, firstly, trifluoromethanesulfonyl chloride, trimethylsilane and solvent are put into a reaction kettle. The reactor is preferably a high-pressure reactor.
[0023] The present invention does not limit the sources of the trifluoromethanesulfonyl chloride and trimethylsilane, which are commercially available. The present invention does not limit its purity, industrial purity and analytical purity can be used.
[0024] In the present invention, the solvent is preferably selected from one or more of n-hexane, tetrahydrofuran, toluene and benzene; more preferably one or more of n-hexane, toluene and benzene. The present invention does ...
Embodiment 1
[0041] Under nitrogen protection, to a 500ml autoclave, set up a mechanical stirrer and a thermometer, 168.5 grams of trifluoromethanesulfonyl chloride (1mol), 74.2g of trimethylsilane (1mol), 2.9 grams of di-tert-butyl peroxide (0.02mol) , 100ml of toluene, the oil bath was heated to an inner temperature of 120°C, and reacted for 3 hours. After the reaction, stir and cool down to room temperature, distill under normal pressure to obtain the crude trifluoromethyl (trimethyl) silane, put the crude product on the rectification column, receive the 53-55 °C fraction, 126.7 grams of trifluoromethyl (trimethyl) silane , GC analysis trifluoromethyl (trimethyl) silane product purity 99.62%, calculated as trifluoromethanesulfonyl chloride, molar yield 88.61%.
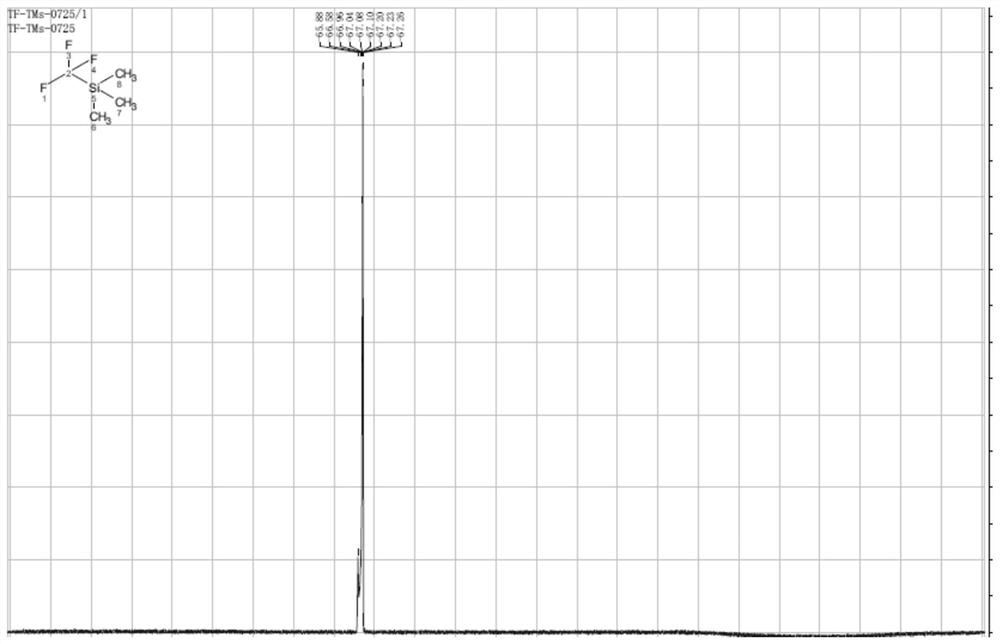
[0042] The trifluoromethyl (trimethyl) silane prepared by the embodiment of the present invention 1 is measured, and the results are as follows figure 1 shown, where figure 1 It is the fluorine spectrum of trifluoromethyl (tr...
Embodiment 2
[0044] Under the protection of nitrogen, in a 500ml autoclave, set up a mechanical stirrer and a thermometer to mix 168.5 grams of trifluoromethanesulfonyl chloride (1mol), 89g of trimethylsilane (1.2mol), 2.9 grams of di-tert-butyl peroxide (0.02mol), 100ml of toluene, the oil bath was heated to an internal temperature of 120°C, and reacted for 3 hours. After the reaction, stir and cool down to room temperature, distill under normal pressure to obtain the crude trifluoromethyl (trimethyl) silane, put the crude product on the rectification column, receive the 53-55 °C fraction, 131.54 grams of trifluoromethyl (trimethyl) silane , GC analysis trifluoromethyl (trimethyl) silane product purity 99.53%, calculated as trifluoromethanesulfonyl chloride, molar yield 92%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com