Synthesis method of glycogen synthase kinase-3 inhibitor for treating plasmodium falciparum causing malaria
A technology for synthesis of Plasmodium falciparum and glycogen, which is applied in drug combination, resistance to vector-borne diseases, organic chemistry, etc., can solve the problem of decreased sensitivity to artemisinin, and achieve the effect of short production cycle and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
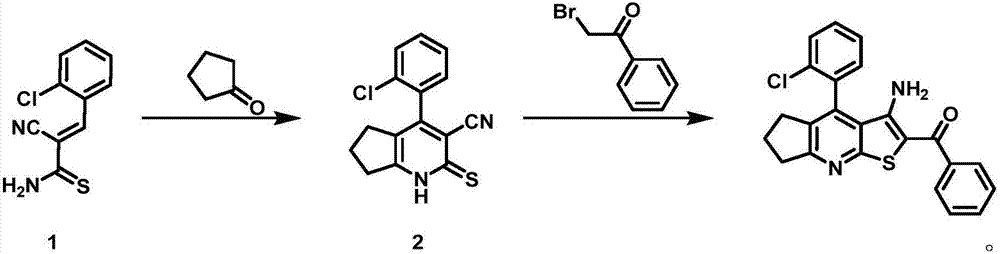
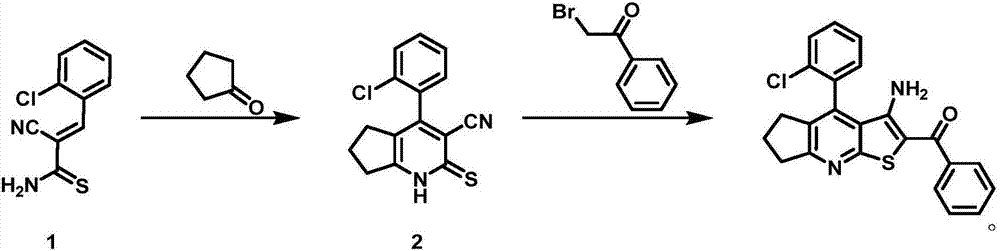
[0020] The synthesis conditions of 4-(2-chlorophenyl)-3-cyano-1,5,6,7-tetrahydro-2H-cyclopenta[b]pyridine-2-thione 2 are:
[0021] Mix cyclopentanone (34.5g), piperidine (12.7g) and dioxane (350mL), heat to 60-80°C and stir for 1-2 hours; add (E)-3-(2 -Chlorophenyl)-2-cyanothioacrylamide 1 (83.0g), stirred for 2-5 hours, cooled to room temperature, filtered to obtain 4-(2-chlorophenyl)-3-cyano-1,5 , 6,7-Tetrahydro-2H-cyclopenta[b]pyridine-2-thione 2 (89.4 g, 84%).
[0022] m.p.:234-238℃; IR(KBr):3225cm -1 (NH),2925cm -1 and 2861cm -1 (aliphatic CH), 2231cm -1 (C≡N); 1 H NMR (DMSO-d6, 500MHz): δ (ppm) = 1.55-1.66 (m, 2H, CH2), 1.90-1.99 (m, 2H, CH2), 2.75-2.85 (m, 2H, CH2), 7.25 ( m,1H,ArH),7.31(m,2H,ArH),7.58(m,2H,ArH),8.02(m,1H,ArH), 14.14(brs,1H,NH); 13 C NMR (DMSO-d6, 125MHz): δ (ppm) = 20.3, 24.8, 27.0, 128.0, 128.9, 130.7, 138.9, 96.3, 113.9, 115.6, 120.1, 140.0, 152.9, 159.0, 175.4.m / z (MS -ESI):287.65[M+1] + .
[0023] Synthesis conditions of 3-amino-4-(2-chl...
Embodiment 2
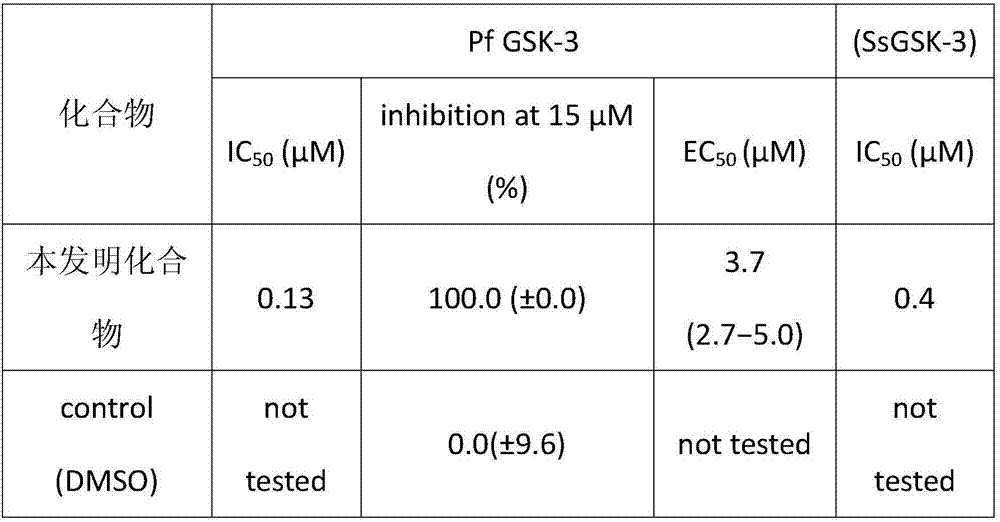
[0026] Example 2 Kinase Analysis
[0027] By mixing the protein with buffer A (pH 7.5, 10mM MgCl 2 , 1 mM EGTA, 1 mM DTT, 25 mM Tris / HCl, pH 7.5, 50 μg / mL heparin, 0.15 mg / mL BSA) in 40 μM GS-1 peptide substrate (YRRAAVPPSPSLSRHSSPHQpSEDEEE, where pS represents phosphorylated serine; Proteogenix, Oberhausbergen, France) at 15 μM γ- 33 Incubate in the presence of P-ATP in a final volume of 30 μL for the measurement of recombinant kinase activity. With the exception of DdGSK3 (30 min at room temperature), 25 [mu]L of the reaction was spotted on Whatman P81 phosphocellulose paper after incubation at 30[deg.] C. for 30 min. The filter was washed 5 times (at least 5 minutes each) in a 1% phosphoric acid solution. Wet filters were counted in the presence of 1 mL of ACS (Amersham) scintillation fluid. Blank values were subtracted and activity was calculated as picomoles of phosphate incorporated during the 30 min incubation. Activity is usually expressed as a percentage of maxim...
Embodiment 3
[0028] Example 3 In Vitro Antimalarial Activity Determination
[0029] Plasmodium falciparum erythroid stage stably expressing the luciferase gene using the hrp2 promoter (NF54:LUC) from a chromosomal locus. These parasites constitutively express high levels of luciferase. Cultures in a total volume of 20 mL were grown in 75 mL tissue culture flasks (Costar brand, NUNC, Denmark). When 3% parasitaemia was reached, the medium was aspirated and 100 μL of 25% hematocrit-cultured parasite multichannel pipettes were transferred to a sterile 96-well plate (100 μL per well). Drug diluted in complete RPMI-1640 containing 1% DMSO (10 µM, 100 µL / well) was added to each well. Each drug was tested in triplicate. As a control (NF54:LUC) three wells without drug in regular medium and another three (NF54:LUC) wells exposed to 1% DMSO only were seeded. An additional 3 wells were inoculated with NF54 "wild type" parasites not expressing luciferase and used as "blanks". Plates were incubate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com