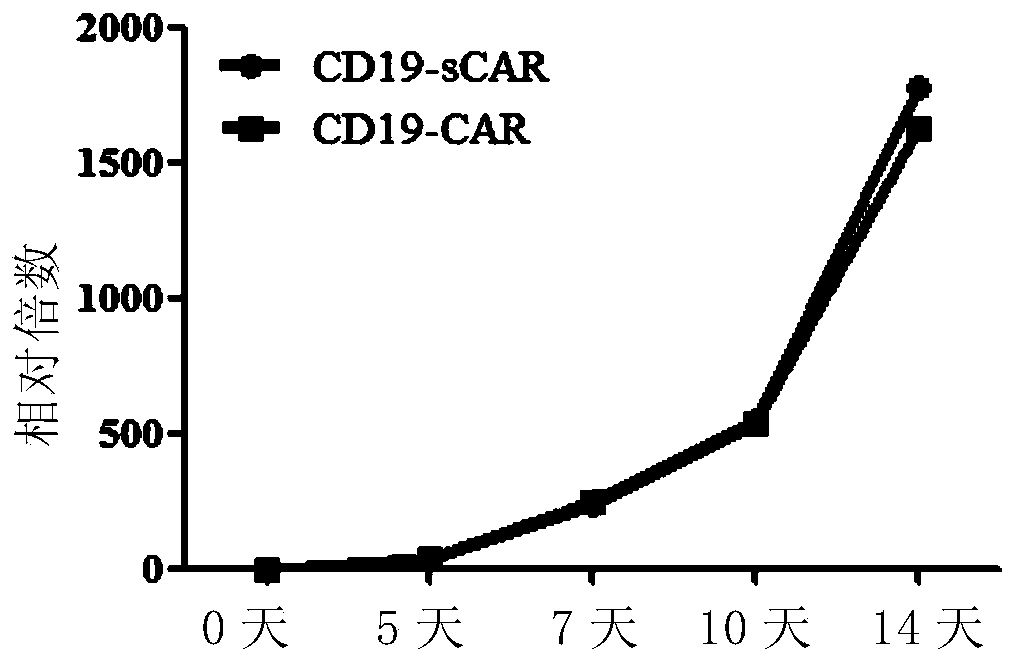
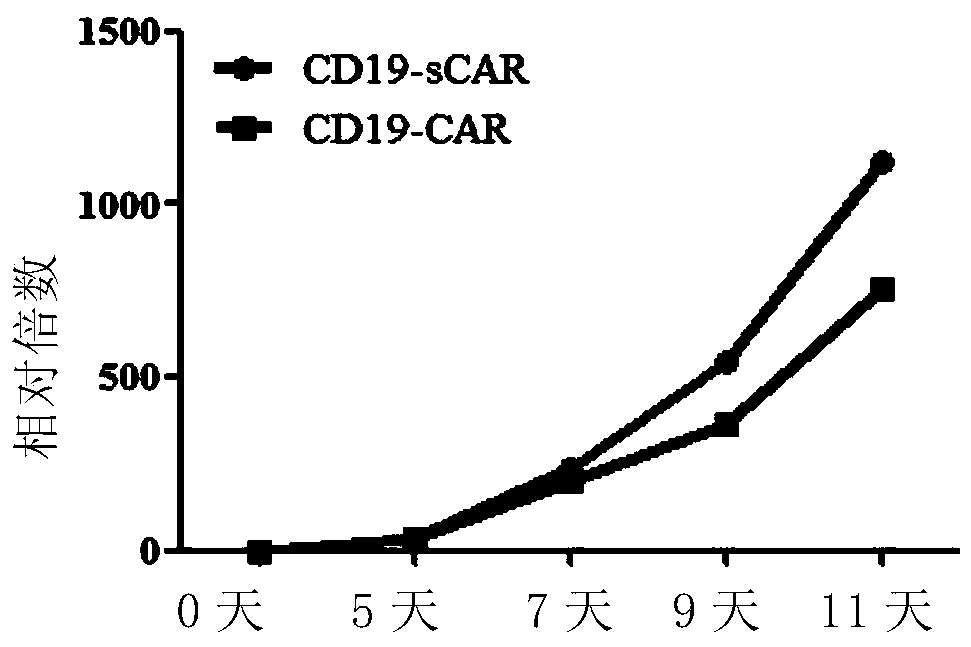
A chimeric antigen receptor for high-efficiency directed amplification in vitro and its application
A chimeric antigen receptor and carrier technology, applied in the fields of genetic engineering and immune cell therapy, can solve problems such as limited progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190] Example 1. Preparation of specific and selective CAR molecules
[0191] Specific primers were designed, and the leader peptide domain (SEQ ID No.1), the coding sequence of the hinge region and the transmembrane region (SEQ ID No. 2) Amplify the coding sequence (SEQ ID No.3) of the intracellular signal transduction domain CD3ζ of the T cell receptor protein CD3 molecule and the intracellular signal activation domain of the CD137 molecule. The specific selectivity domain in the CAR molecule is derived from the 95th-104th amino acid coding sequence (SEQ ID No.4) of the C-terminal domain of human nucleoprotein La / SS-B, which is prepared by chemical synthesis and inserted into Between the VL and VH domains of the humanized single-chain antibody ScFv coding region in the CAR molecule. The structure of the CAR is F 0 -F 1 -L 1 -Z-L 2 -F 2 -H-TM-C-CD3ζ, the amino acid sequence is shown in SEQ ID NO.:8. The above-mentioned different coding sequences were spliced and amp...
Embodiment 2
[0192] Example 2. Construction of Chimeric Antigen Receptor Expression Vector
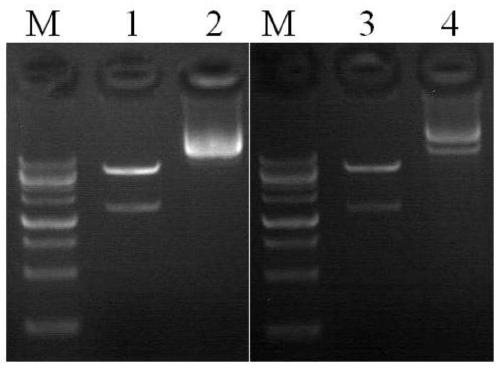
[0193] The CAR coding sequence (SEQ ID NO.: 7) in Example 1 was cloned into the lentiviral expression vector pLenti-CMV using molecular cloning technology. In order to compare the advantages of the modified CAR molecule in the present invention in preparing chimeric antigen receptor T cells, a conventional CAR molecule targeting CD19 antigen (amino acid sequence shown in SEQ ID NO.: 9, patent No. : CN103492406A) as a control, a CD19-CAR virus expression vector was constructed. The above-mentioned lentiviral expression vector is used together with the viral packaging helper plasmid, the plasmid psPAX2 encoding the viral nucleocapsid proteins Gag / Pol and Rev, and the plasmid pVSVG encoding the viral envelope protein, for the subsequent preparation of different CAR gene-encoded lentiviruses. figure 1 It is the result of identifying viral expression vectors carrying different CAR protein coding sequen...
Embodiment 3
[0195] Example 3. Preparation of CAR gene-encoded virus
[0196] HEK193T was used as packaging cells for the preparation of CAR gene-encoded viruses. HEK293T cells in the logarithmic growth phase were digested, centrifuged at 800 rpm for 5 minutes, and the culture medium was discarded, and then resuspended with DMEM medium (Gbico Company) containing 10% FBS (Gbico Company). After cell counting, adjust the density of the cell suspension to 3.6 × 10 6 / ml, placed in a 37°C cell culture incubator for use. The transfection of the virus packaging plasmid used Lipofectamine 3000 kit (ThermoFisher Company), and operated according to the instructions of the kit. Mix the three plasmids required for lentiviral packaging, including viral vectors containing different CAR genes and the two helper plasmids mentioned in Example 2, with Lipofectamine 3000 according to the recommended ratio in the instructions to prepare a DNA-liposome complex, at room temperature Let stand for 15 minutes. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com