Bis(morpholinoalkoxy)quinazoline derivatives and their use in antitumor
A technology of morpholinyl alkane and quinazoline, which is applied in the field of bisquinazoline derivatives or their pharmaceutically acceptable salts, and can solve problems such as difficult tumor treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
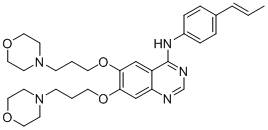
[0068] Example 1: 4-[4-(E)-(propen-1-yl)anilino]-6,7-bis[3-(4-morpholinyl)propoxyl]quinazoline (A) synthesis
[0069] (1) Preparation of 3,4-bis(3-chloropropoxy)benzonitrile
[0070]
[0071] Sequentially add anhydrous K 2 CO 3 (0.03mol), 0.6mL of PEG-400 and 30mL of acetonitrile, 1,3-bromochloropropane (0.06mol), heated to 60 ° C, and added 3,4-dihydroxybenzonitrile (0.01mol) in six times, Continue the reaction, TLC tracking [developer: ethyl acetate: petroleum ether (1:4, V / V)] to the completion of the reaction, the reaction time is about 3h. Stop the reaction and filter while hot to remove K 2 CO 3 , the filter cake was washed three times with hot acetonitrile, and the filtrate was distilled to recover acetonitrile and 1,3-bromochloropropane to obtain a brown oily residue, which was separated by silica gel column chromatography [ethyl acetate:petroleum ether (1:6, V / V)] to obtain white solid 3,4-bis(3-chloropropoxy)benzonitrile (yield 86.4%).
[0072] (2) Prepara...
Embodiment 2
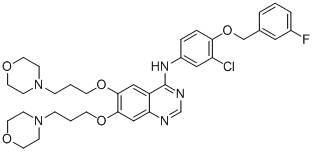
[0091] Example 2: 4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6,7-bis[3-(4-morpholinyl)propoxy]quinazoline (B) Synthesis
[0092] Step (1)~(4) is the same as embodiment 1,
[0093] (5) Preparation of 4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6,7-bis(3-chloropropoxy)quinazoline
[0094]
[0095] According to the method of step 5 of example 1, 4-(E)-aminophenylpropene in step 5 of example 1 was replaced with 3-chloro-4-(3-fluorobenzyloxy)aniline (1.10mmol) to prepare a white Solid 4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6,7-bis(3-chloropropoxy)quinazoline (yield 93.8%).
[0096] (6) 4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6,7-bis[3-(4-morpholinyl)propoxy]quinazoline (B) preparation
[0097]
[0098] According to the method of step 6 of Example 1, with 4-[3-chloro-4-(3-fluorobenzyloxy)anilino]-6,7-bis(3-chloropropoxy)quinazoline (0.50mmol ) instead of 4-[4-(E)-(propene-1-yl)anilino]-6,7-bis(3-chloropropoxy)quinazoline in Step 6 of Example 1, to prepare a light yellow s...
Embodiment 3
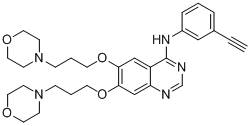
[0103] Example 3: Synthesis of 4-(3-ethynylanilino)-6,7-bis[3-(4-morpholinyl)propoxy]quinazoline (C)
[0104] Steps (1)-(4) are the same as in Example 1.
[0105] (5) Preparation of 4-(3-ethynylanilino)-6,7-bis(3-chloropropoxy)quinazoline
[0106]
[0107] According to the method of step 5 of example 1, 4-(E)-aminophenylpropene in step 5 of example 1 was replaced with 3-ethynylaniline (1.10mmol) to prepare a white solid 4-(3-ethynylaniline) -6,7-bis(3-chloropropoxy)quinazoline (yield 90.6%).
[0108] (6) Preparation of 4-(3-ethynylanilino)-6,7-bis[3-(4-morpholinyl)propoxy]quinazoline (C)
[0109]
[0110] According to the method of step 6 of embodiment 1, replace 4- [4-(E)-(propen-1-yl)anilino]-6,7-bis(3-chloropropoxy)quinazoline was prepared as light yellow solid C (yield 71.5%).
[0111] m.p.:91.5–92.7℃; HRMS(C 30 h 37 N 5 o 4 )m / z[M+H] + :532.2920 (calculated value: 532.2924).
[0112] 1 H-NMR (600MHz, DMSO-d 6 )δ (ppm): 9.51 (s, 1H), 8.49 (s, 1H), 8.02–8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com