Nicorandil freeze-dried powder injection and preparation method thereof
A technology for freeze-dried powder injection and nicorandil, which is applied in the field of nicorandil freeze-dried powder injection for injection and its preparation, can solve problems such as instability, and achieves improved stability, delayed degradation speed, and improved product quality. quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
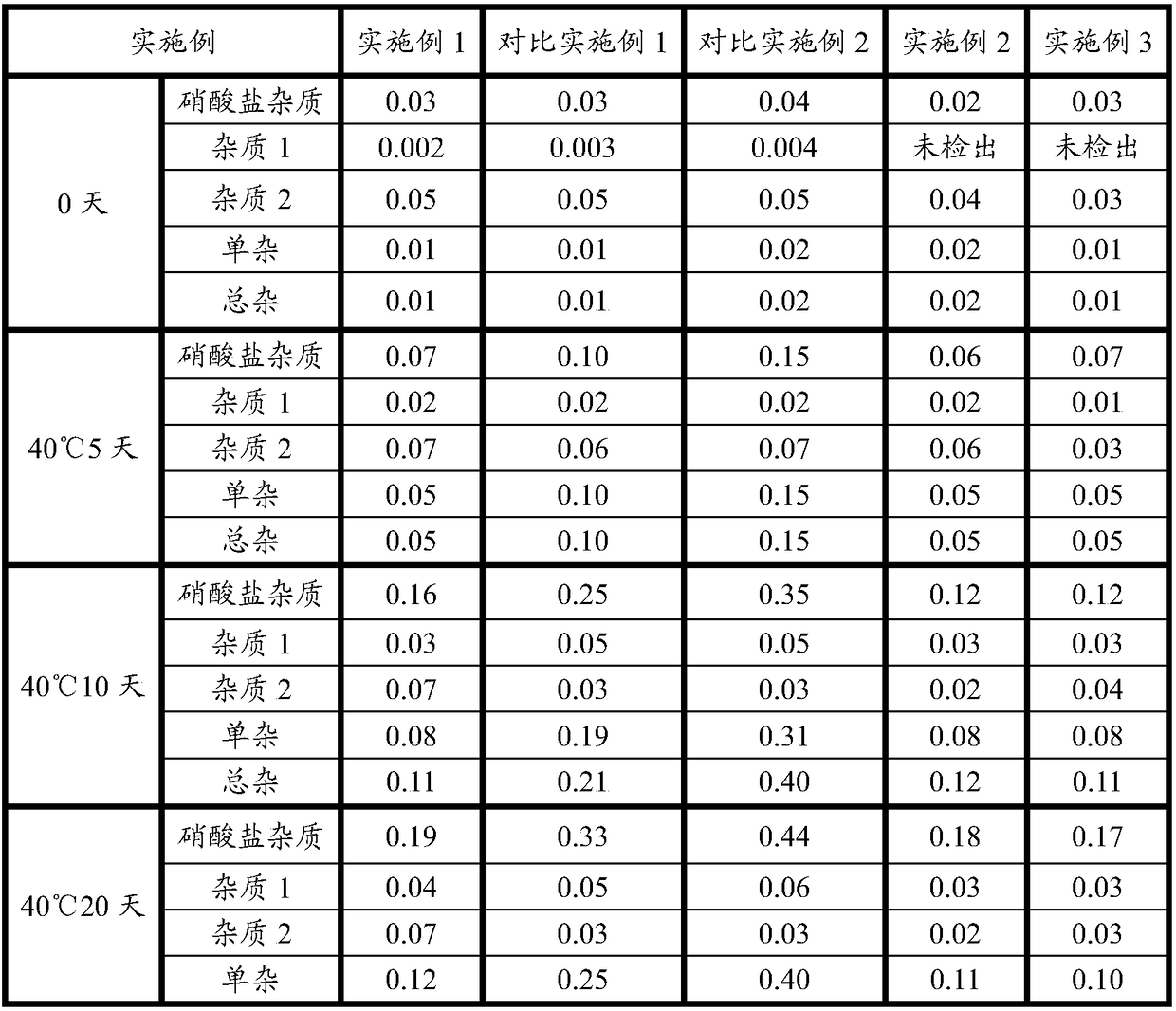
Examples
Embodiment 1
1ml
[0022] Preparation:
[0023] 1. Preparation of preparation solution: Dissolve mannitol and nicorandil sequentially in 80% of the prescribed amount of water for injection. After dissolving, adjust the pH to 7.0-8.0 with sodium citrate solution, and dilute to volume with water for injection.
[0024] 2 The preparation solution is sterilized by filtration, filled with a filling volume of 1 ml, half-stoppered, and placed in a freeze dryer for freeze-drying.
[0025] 3 Freeze-dry according to the following drying procedure: pre-freezing stage: cool down the plate layer to -40°C, maintain for 3h, then raise the temperature to -3°C, maintain for 1h, then cool down to -40°C, maintain for 1h, and then raise the temperature to -3°C , maintain for 1h, then cool down to -40°C, maintain for 1h, and enter the sublimation stage; the vacuum degree of sublimation is 200ubar, the sublimation temperature is -20°C, maintain for 10h, then raise the temperature to -10°C, maintain for 3h...
Embodiment 2
2ml
[0043] Preparation:
[0044] 1. Preparation of preparation: Dissolve mannitol and nicorandil sequentially in 80% of the prescribed amount of water for injection. After dissolving, adjust the pH of the sodium citrate solution to 7.0-8.0, and dilute the water for injection.
[0045] 2 The preparation solution is sterilized by filtration, filled with a filling volume of 2 ml, half-stoppered, and placed in a freeze dryer for freeze-drying.
[0046] 3 Freeze-dry according to the following drying procedure: pre-freezing stage: cool down the plate layer to -40°C, maintain for 3h, then raise the temperature to -3°C, maintain for 4h, then cool down to -40°C, maintain for 1h, then raise the temperature to -3°C , maintain for 4 hours, then cool down to -40°C, maintain for 1h, and enter the sublimation stage; the vacuum degree of sublimation is 200ubar, the sublimation temperature is -20°C, maintain for 10h, then raise the temperature to -10°C, maintain for 3h, and then raise ...
Embodiment 3
8ml
[0050] Preparation:
[0051] 1. Preparation of preparation solution: Dissolve mannitol and nicorandil sequentially in 80% of the prescribed amount of water for injection. After dissolving, adjust the pH to 7.0-8.0 with sodium citrate solution, and dilute to volume with water for injection.
[0052] 2 The preparation solution is sterilized by filtration, filled with a filling volume of 8 ml, half-stoppered, and placed in a freeze dryer for freeze-drying.
[0053] 3 Freeze-dry according to the following drying procedure: pre-freezing stage: cool down the plate layer to -40°C, maintain for 3h, then raise the temperature to -3°C, maintain for 4h, then cool down to -40°C, maintain for 1h, then raise the temperature to -3°C , maintain for 4 hours, then cool down to -40°C, maintain for 1h, and enter the sublimation stage; the vacuum degree of sublimation is 200ubar, the sublimation temperature is -20°C, maintain for 10h, then raise the temperature to -10°C, maintain for 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com