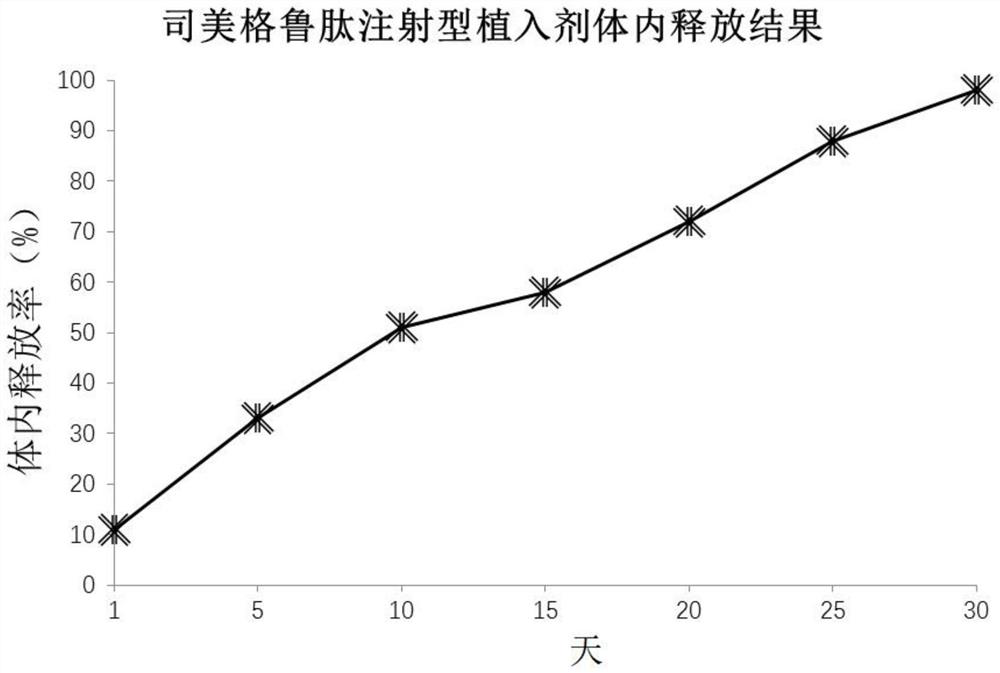
Smeglutide implant and preparation method thereof
An implant and organic solvent technology, applied in the field of drug preparation, can solve the problems of inconvenient drug storage, increased drug risk, tissue damage, etc., and achieve the effects of improving bioavailability, improving convenience, and increasing stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The present invention provides a semaglutide implant and a preparation method thereof, and those skilled in the art can learn from the content of the present invention and appropriately improve process parameters to achieve. It should be particularly pointed out that all similar substitutions and modifications apparent to those skilled in the art are deemed to be included in the present invention. The method and application of the present invention have been described through the preferred embodiments, and it is obvious that relevant persons can make changes or appropriate changes and combinations of the methods and applications described herein without departing from the content, spirit and scope of the present invention to achieve and Apply the technology of the present invention.
[0032] The implant preparation provided by the present invention and the raw materials, auxiliary materials and reagents used in the preparation method and use thereof can be purchased fro...
Embodiment 1
[0034] Example 1: Preparation of Semaglutide Injection Subcutaneous Implant
[0035] The prescription of the present embodiment is as follows (1000):
[0036] Element Dosage Semaglutide sodium salt 4.005g (equivalent to semaglutide 4g) N-(8-(2-Hydroxybenzoyl)amino)octanoic acid (NAC) 10g PLA (M=20000 Daltons) 40g Poloxamer 188 120g Sorbitol 8g
[0037] PLA (M=20000) was completely dissolved in ethyl acetate to prepare a 5% ethyl acetate solution of PLA, and then semaglutide sodium salt and excipient N-(8-(2-hydroxybenzene) were added to it. Formyl)amino)caprylic acid (NAC) was fully stirred to prepare a PLA ethyl acetate solution containing semaglutide and N-(8-(2-hydroxybenzoyl)amino)octanoic acid (NAC) at 25°C. PLA microcapsules of semaglutide sodium were prepared by spray drying under the pressure of 0.5 MPa.
[0038] Take 20 ml of water for injection and cool it to 5°C, add poloxamer 188 at 2-10°C, mix well, and let it stand...
Embodiment 2
[0042] Example 2: Preparation of Semaglutide Injection Subcutaneous Implant
[0043] The prescription of the present embodiment is as follows (1000):
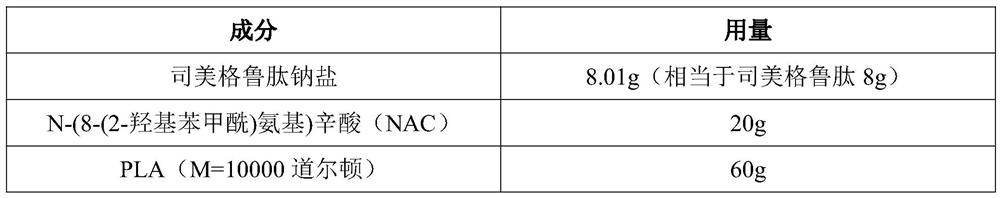
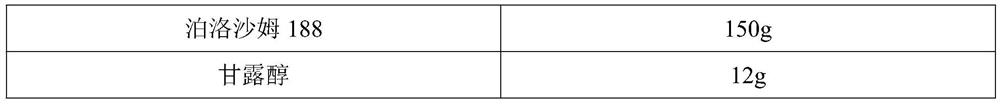
[0044] Element Dosage Semaglutide sodium salt 4.005g (equivalent to semaglutide 4g) N-(8-(2-Hydroxybenzoyl)amino)octanoic acid (NAC) 20g PLA (M=10000 Daltons) 40g Poloxamer 188 120g Mannitol 8g
[0045] PLA (M=10000) was completely dissolved in ethyl acetate to prepare a 5% PLA in ethyl acetate solution, and then semaglutide sodium salt and excipient N-(8-(2-hydroxybenzene) were added to it. Formyl)amino)octanoic acid (NAC), stir well, and prepare a PLA ethyl acetate solution containing semaglutide and excipient N-(8-(2-hydroxybenzoyl)amino)octanoic acid (NAC). The PLA microcapsules of semaglutide sodium were prepared by spray drying at 25° C. under a pressure of 1.0 MPa.
[0046] Take 20 ml of water for injection and cool it to 5°C, add poloxamer 188 at 2-10°C, mix well, and let...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com