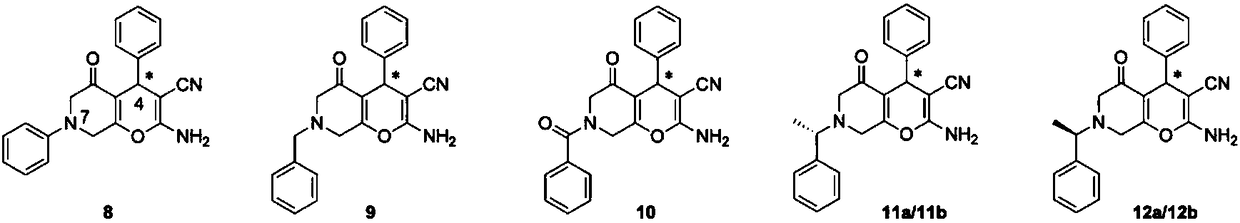
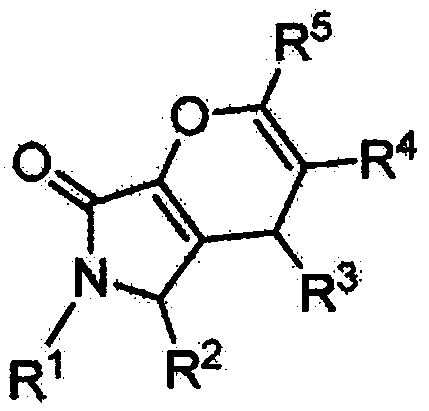
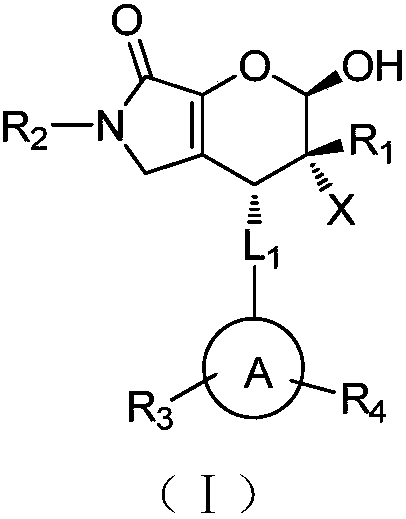
Halogenated dihydropyran pyrrolidone compound as well as preparation method and application thereof
A technology of halogenated dihydropyranopyrrolone compounds and compounds, which is applied in the field of halogenated dihydropyranopyrrolone compounds and their preparation, and can solve problems such as halogenated dihydropyranopyrrolone compounds that have not been reported , achieve the effect of broad market application prospect, high yield and mild reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1, the preparation of compound of the present invention
[0040] raw material
[0041] Quinidine squaramide catalyst: the structure is Purchased from Daicel Pharmaceutical Chiral Technology (Shanghai) Co., Ltd.
[0042] General synthetic route:
[0043]
[0044] Wherein, X is fluorine, chlorine, bromine, iodine; L 1 Is none or vinyl; ring A is naphthalene ring, benzene ring, thiophene ring; R 1 is benzyl, C 1 -C 8 Alkyl or (CH 2 ) n -O-Bn; n is an integer from 1 to 5; R 2 Be benzyl, allyl, p-methoxybenzyl; R 3 , R 4 independently selected from hydrogen, fluorine, chlorine, bromine, iodine, C 1 -C 3 Alkyl, nitro, methoxy.
[0045] The following compounds were prepared:
[0046]
[0047]
[0048]
[0049] 1. Preparation of compound 4a
[0050]
[0051] ①Take a 150mL round bottom flask, measure 30mmol of benzylamine, 10mL of absolute ethanol, and an equivalent amount of ethyl acrylate, and stir at room temperature for 16h. Weigh 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com