Taxol and CDKS kinase inhibitor anti-tumor combined pharmaceutical composition
A kinase inhibitor, paclitaxel technology, applied in the field of chemical medicine, can solve problems such as the ineffectiveness of anticancer agents, and achieve the effects of reducing clinical dosage, reducing toxic side effects, and good anticancer efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
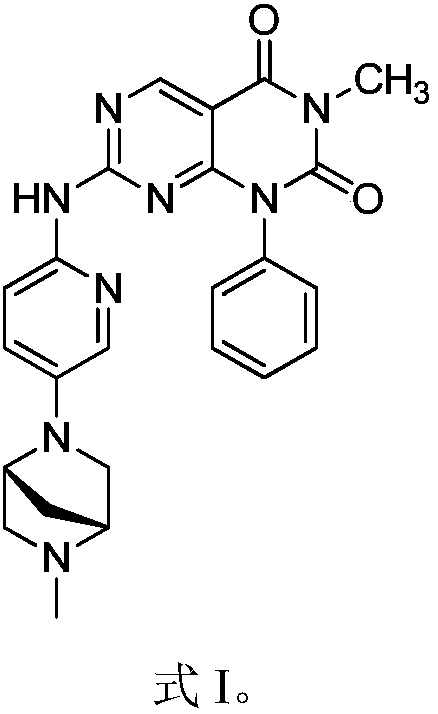
[0018] Example 1 1-phenyl-3-methyl-7-(5-(5-methyl-2,5-diazabicyclo[2.2.1]hept-2-yl)pyridin-2-ylamino ) Synthesis of pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione
[0019]
[0020] Step 1 Synthesis of 2-amino-5-(5-methyl-2,5-diazabicyclo[2.2.1]hept-2-yl)pyridine
[0021]
[0022] Weigh 2.24g of 5-methyl-2,5-diazabicyclo[2.2.1]heptane into a reaction flask, add 50ml of DMF / water mixed solvent with a volume ratio of 1:1 to dissolve, add 0.52g of 2- Amino-5-chloropyridine was reacted at 100°C for 8 hours. After the reaction was completed, 20ml of water was added, extracted with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain the title compound.
[0023] LC-MS m / z:[M+H] + =205.
[0024] Step 2 1-phenyl-3-methyl-7-(5-(5-methyl-2,5-diazabicyclo[2.2.1]hept-2-yl)pyridin-2-ylamino) Synthesis of pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione
[0025] Using the product obtained in Step 1, 2,4-dichloro-5-nitropyrimidine, and ...
Embodiment 2
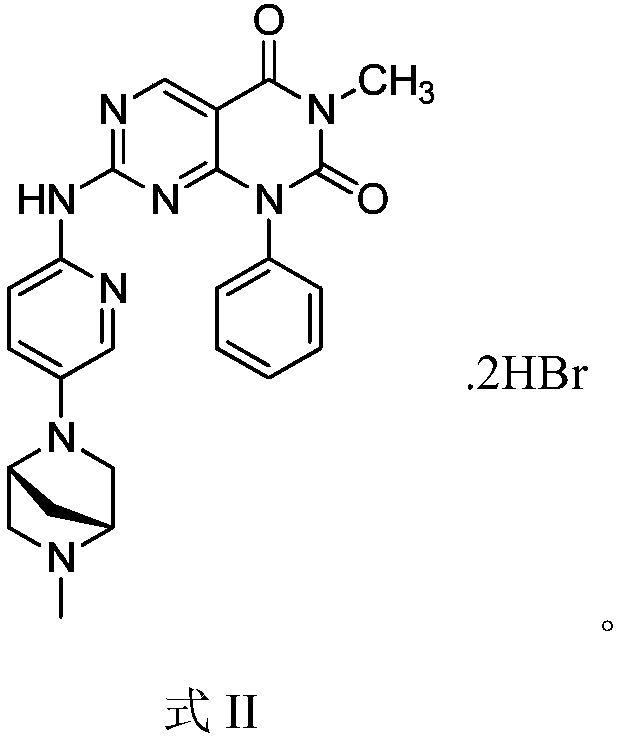
[0028] Example 2 1-phenyl-3-methyl-7-(5-(5-methyl-2,5-diazabicyclo[2.2.1]hept-2-yl)pyridin-2-ylamino ) Synthesis of pyrimido[4,5-d]pyrimidine-2,4(1H,3H)-dione hydrobromide
[0029]
[0030] Weigh 15g of the compound of Example 1 into a reaction flask, add 50ml of dichloromethane to dissolve, add 5.9g of hydrobromic acid, heat up to 45°C and stir for 0.5h, after cooling to room temperature, evaporate the solvent under reduced pressure to obtain the title compound.
experiment example 1
[0031] Experimental Example 1 Stability Experiment
[0032] Weighed 4 copies of 1 g of the compounds of Examples 1 to 3, and placed them under the conditions of light 4500Lx, RH70% 75°C, RH70% 60°C and RH70% room temperature for 1 month. The experimental results are shown in Table 1.
[0033] Table 1
[0034]
[0035] Experimental results show that the compounds of Examples 2 and 3 of the present invention have very high stability.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com